
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:* OWLY2 |Online
Schedu x My Home
ssignment/takeCovalentActivity.do?locator%3Dassignment-take
C MasteringAandP: L.
G what is the cell wall..
G Mary was in a terrib..
Dimensional Analy..
L.
[Review Topics
TReferences
Use the Referemces ta acces impartant vaees if needed far this question.
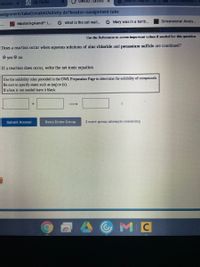
Does a reaction occur when aqueous solutions of zinc chloride and potassium sulfide are combined?
O yes O no
If a reaction does occur, write the net ionic equation.
Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds.
Be sure to specify states such as (aq) or (s).
If a box is not needed leave it blank.
Submit Answer
Retry Entire Group
2 more group attempts remaining
MC
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 6. lead (II) nitrate + sodium chromate 7. nickel (II) nitrate + sodium hydroxidearrow_forwardVisited [Review Topics] [References] Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank. Write the net ionic equation for the following molecular equation. MnSO4(aq) + Na₂CO3(aq) -MnCO3(s) + Na₂SO4(aq) + Submit Answer ->> Retry Entire Group + 8 more group attempts remaining Prevarrow_forward1. Would a precipitate form from a reaction between sodium carbonate and calcium chloride solutions? Write the balanced, overall ionic, and net ionic equations for this reaction and indicate any solids (if they form) lon Solubility Exceptions = slightly soluble %3D NO3- soluble none CIO4 soluble none except Ag+, Hg22+, *Pb2+ except Agt, Нg2+, "РЬ2+ except Agt. Hg2+, "РЬ2+ except Ca2+, Ba2+, Sr2+, Hg2+, Pb2+, Ag+ CH soluble soluble Br- soluble SO 2- soluble CO32- insoluble except Group IA and NH4+ insoluble except Group IA and NH4+ insoluble except Group IA, *Ca2+, *Ba²+, *Sr2+ insoluble except Group IA, IIA and NH4* PO23 OH- S2- Nat soluble None soluble None None K+ NH4 solublearrow_forward
- Timely matter!arrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardPls help ASAP ON ALL ASKED QUESTIONS PLS PLSarrow_forward
- please answer questionarrow_forwardBackground-info: https://drive.google.com/file/d/1G7sPTuESIgWk9wpRdqAnFcsBmOlbBfLv/view?usp=sharing Question: In carrying out Part 1 of this experiment, assume an exces of HCI(aq) reacts with 0.296 g of Mgls). If the volume of the final solution is 69.0 mL, what is the stoichiometric concentration (in mol/L) of MgCl2laq) after the reaction is complete? Report your answer to the correct number of significant figures and only include the numerical answer (no units).arrow_forwardGive a clear handwritten answer with explanationarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY