
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
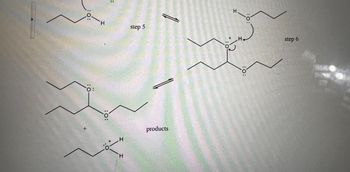
Transcribed Image Text::0:
: 0:
:0:
H
step 5
H
ö:
+
H
products
:0:
:0
H
step 6
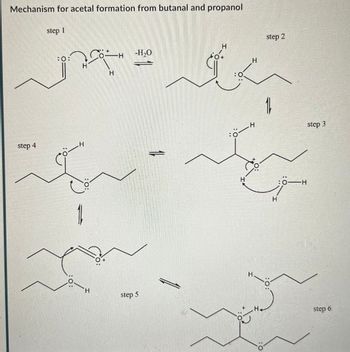
Transcribed Image Text:Mechanism for acetal formation from butanal and propanol
step 1
step 4
:0
.H
H
H
-H₂O
H
H
H
step 5
ة
step 2
16
H
H
..
:0-H
H.
H+
:0
:0:
step 3
step 6
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Mechanism: A reaction mechanism for the following reaction is shown below. H+ CEN CEN: N-H || -C-OH H₂O, H* Step 1 Step 3 N-H C-OH C=N-H H₂O Step2 .C=N-H H-O-H Semse anls a) The overall reaction is an example of b) Step 1 is c) Step 3 is d) Draw in the curved arrows for each step. e) Identify the nucleophiles and electrophiles where appropriate. f) Fill in the reaction energy diagram. C=N-H H₂O: rate determining step E个 reaction progress →arrow_forward1. What is final product? 2. What are the 4 reactions & steps in mechanism (show product and mechanism steps)? 3. It is kinetic or thermodynamic?arrow_forwardWhich of the following represents an enolate? of I. O I. only II. and III. O III. only O I. and III. II. III.arrow_forward
- Draw curved arrows to show how the reaction forms the product .arrow_forwardA set of three nucleophilic displacement reactions is shown below: CH3 CH3 CH3 CH3 CH3 CH,CHCH,CH,CCH,CI CH,CHCH,CH,CCH,CH, CH,CHCHCH,CHCH;CH,CH, CH;CHCHCH,CH2CH,CH,CH3 CH3 CI C H2O, HCO2H SN1 reaction Which reaction (A, B, or C) proceeds the fastest? Which reaction (A, B, or C) proceeds the slowest?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY