
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
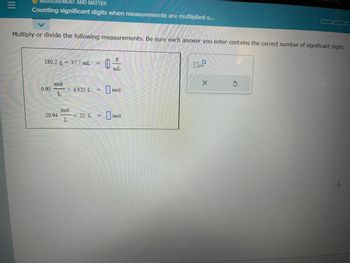
Transcribed Image Text:**Topic: Counting Significant Digits When Measurements Are Multiplied or Divided**
**Instructions:**
Multiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant digits.
**Problems:**
1. \( \frac{180.2 \, \text{g}}{37.7 \, \text{mL}} = \, \_\_\_\_\_\_ \, \frac{\text{g}}{\text{mL}} \)
2. \( 0.93 \, \frac{\text{mol}}{\text{L}} \times 4.625 \, \text{L} = \, \_\_\_\_\_\_ \, \text{mol} \)
3. \( 20.94 \, \frac{\text{mol}}{\text{L}} \times 25. \, \text{L} = \, \_\_\_\_\_\_ \, \text{mol} \)
**Tools Provided:**
- A clear input box for each problem to enter your answer.
- A button to indicate powers of ten (\( \times 10 \)) if needed.
- Options to clear or reset your answer.
**Note:** Ensure that your answers respect the rules of significant digits in scientific calculations.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1.0936 yards 1 pound 453.59 grams 16 ounces 1 meter 1 ton 2000 pounds 907.184 kilograms 2.54 centimeters 1 inch 2204.6 pounds 1000 kilograms 1 kilometer 0.62137 miles 1 metric ton = 1 mile 5280 feet The density of a sample of an unknown solid is 17.7 g/cm3. Convert this density to to units of Ib/ft3 You don't need to include units, but, pay attention to significant figures! .? To answer in scientific notation, use the following convention: 1.0 x 10-3 is written as 1.0E-3arrow_forwardWhich numbers are exact (and therefore have an unlimited number of significant figures)?a. p = 3.14b. 12 in = 1 ftc. EPA gas mileage rating of 26 miles per gallond. 1 gross = 144arrow_forward5. Perform the following unit conversions. Show the setups for each conversion (cancel units). Give your answers to the correct number of significant figures. a) 275 yd to m b) 0.0053 gall to L c) 5.788 x 108 µg to lb d) 355 m/s to miles/hrarrow_forward
- Convert 8.40 g/cm2 to mg/mm2. Round your answer to 1 decimal place. No units needed.arrow_forwardPlease don't provide handwritten solution .....arrow_forwardCarry out the following operations as if they were calculations fromexperimental data and express each answer in the correct units and with thecorrect number of significant figures.a. 5.6792 m + 0.6 m + 4.33 m =b. 3.70 g - 2.9133 g =c. 4.51 cm X 3.6666 cm =d. (22.4 g + 102.7 g + 0.005 g) / 5.478 ml =arrow_forward
- 4.-Convert the following numbers to scientific notation, use prefixes and use only 2 significant figures 52 X 10° g= 52Mg = 52 megagrams Example 52,000,000 g 0.0000003 g a. b. 26198151 L 1546 m C.arrow_forward2. An object has a volume of 0.0012 m3 and a mass of of 2.26 kg. Calculate the density of the object in g/cm3.arrow_forwardThe mass of a certain atom is 2.086 x 10°22 g. This is the same mass as 2.086 x 10-16 mg. 2.086 x 10-25 kg. 2.086 x 10-28 Hg. O 2.086 x 10-31 ng.arrow_forward
- 1. Why are units so important in measurement? Giving examples of measurements with no units should help. 2. What does it mean to say that 2 expressions are equivalent?arrow_forwardM Gmail YouTube = Maps O MEASUREMENT Counting significant digits when measurements are multiplied o... Multiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant digits. 764.45 g 0.39 mL = 786.4 mol 48.853 L 7.81 mol L X 4.25 L = = 0 g mL mol 0- mol x10 X Ś 0/5 Olivi.arrow_forwardQuestion 2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY