
Physical Chemistry
2nd Edition
ISBN: 9781133958437
Author: Ball, David W. (david Warren), BAER, Tomas
Publisher: Wadsworth Cengage Learning,
expand_more
expand_more
format_list_bulleted
Question
Helpppsppspspppspspspslslsls
A. Are the coefficients from balanced equations
B. Do not appear in a mass action expression
C. Can be expressed in terms of concentrations or partial pressures
D. Is signified by brackets
E. Must be expressed in terms of concentrations
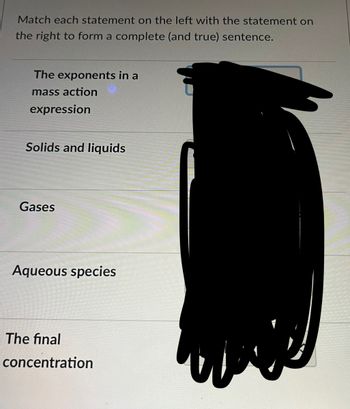
Transcribed Image Text:Match each statement on the left with the statement on
the right to form a complete (and true) sentence.
The exponents in a
mass action
expression
Solids and liquids
Gases
Aqueous species
The final
concentration
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- From the data in Table 14.2, predict B for DCl D is 2H.arrow_forwardRank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on. substance A B U D chemical symbol, chemical formula or Lewis structure H H 1 | H-C C 1 I I H H H Ar H I Ag : 0: C-O-H :0: ||| H-C-C-C-H 44 boiling point ✓ (Choose one) 1 (highest) 2 W N 3 4 (lowest) (Choose one) ↑ (Choose one) (Choose one) ↑arrow_forwardRank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on. substance A B C D chemical symbol, chemical formula or Lewis structure H :0: I | H H-C-C-0-C-H F₂ H :O: ||| : 0: H H 1 .. H H 1 HIC-C-N-C-H H H :NEN 0: boiling point (Choose one) ✓ (Choose one) ✓ (Choose one) (Choose one) Xarrow_forward
- nk the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling nt, choose 2 next to the substance with the next highest boiling point, and so on. substance A B C D chemical symbol, chemical formula or Lewis structure NO :O: H HIC C-H 1 H N₂ :0: || .. HIC N-H 1 H boiling point ✓ (Choose one) 1 (highest) 2 3 4 (lowest) (Choose one) ✓ (Choose one) ✓ X Śarrow_forwardRank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on. substance A B C D H chemical symbol, chemical formula or Lewis structure H :0: 44 - C- C-H H - H 1 C I Ar H H | | - C - | 1 H H H Ag H CIO : 0: H boiling point ✓ (Choose one) 1 (highest) 2 3 4 (lowest) (Choose one) ✓ (Choose one) ✓arrow_forward7.84 Which of the following molecules is least likely to actually exist OF4,SF4,SeF4 , or TeF4 ? Why?arrow_forward
- The structure of pyridine is When a proton becomes bonded to the nitrogen atom by way of its unshared electron pair, the result is _____________________________arrow_forwardCalculate the lattice energy of KCI(s) using the following thermodynamic data (all data is in kJ/mol) K(s) AHsublimation = 69 kJ/mol K(g) Ionization energy = 399 kJ/mol CI-CI(g) Bond energy = 223 kJ/mol Cl(g) Electron affinity = -369 kJ/mol KCI(s) AH°r= -457 kJ/mol kJ/molarrow_forward11111111111111122222222222222222222220000000000000000000000000000000444444444444444444444444444 pls answerarrow_forward
- @- Please I want answer for this question by typing it. Many Thanksarrow_forwardAll I'm givenarrow_forwardRank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on. substance A B U H H C - :NEN 0: chemical symbol, chemical formula or Lewis structure H :0: | || C H HIC - H F₁ H :0: | || с C - : 0: H -0. - C N- I H H C-H H 1 C —E H - H boiling point ✓ (Choose one) C 1 (highest) 2 3 4 (lowest) (Choose one) ↑ (Choose one) C olo 18 Ararrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning