
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
HW - ORG CHEM please illustrate answer onto image
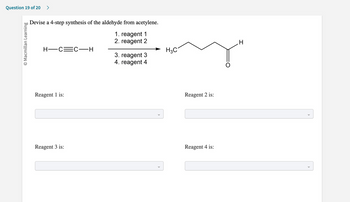
Transcribed Image Text:Question 19 of 20 >
Ⓒ Macmillan Learning
Devise a 4-step synthesis of the aldehyde from acetylene.
1. reagent 1
2. reagent 2
H-C=C-H
Reagent 1 is:
Reagent 3 is:
3. reagent 3
4. reagent 4
H3C
Reagent 2 is:
Reagent 4 is:
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- M eq eq q eq eq eq eq eq eq eq eq Apple Google Disney ESPN Yahoo! Biomedical Careers Program Apple ☆ B Submit Answer prod03-cnow-owl.cengagenow.com iCloud Yahoo Images Bing Google Wikipedia Facebook Twitter LinkedIn The Weather Channel G D2L b COWLv2 |... b C Retry Entire Group PEOPLESOFT North Central D2L [Review Topics] [References] Use the References to access important values if needed for this question. For lead, Pb, the heat of vaporization at its normal boiling point of 1740 °C is 177.8 kJ/mol. The entropy change when 2.08 moles of liquid Pb vaporizes at 1740 °C, 1 atm is D2L 4 more group attempts remaining J/K. C G Yelp TripAdvisor M G C + 88arrow_forward92% D Sun 3:04 PM OneLogin B McGraw-Hill Can O Question 1- 8.4 M MHE Reader B McGraw-Hill Cam x A ALEKS - Esther C x -> A www-awa.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IBcn9hvCfbYq_fi3Zsn8H2oW_5PTMiOacVC-TPIQJ5aqZ8Tg8iSQs6Rzj49leNgLwH_Jfre.. ☆ -pps M Gmail O YouTube O Maps BC Broward College |.. O Sample Source An.. O New Tab G What does Duckw. E Untitled documen. B Reading List O KINETICS AND EQUILIBRIUM Using reactant reaction order to predict changes in initial rate Esther A certain reaction is first order in H, and first order in I,. Use this information to complete the table below. Be sure each of your answer entries has the correct number of significant digits. [4] [1] initial rate of reaction 1.25 M 1.39 M 15.0 M/s do 0.431 M 1.39 M 2.06 M 0.844 M Check Explanation O 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use I Privacy | Accessibility 642 4 MacBook Airarrow_forwardFree Article Rewrite.. O Essaylyper J Turnitin A ALEKS Assessme. +| 回の 會 3 / 8 100% JF 20) 20) Identify the compound that is NOT a major air pollutant. A) nitrogen oxides B) carbon monoxide C) ozone D) sulfur oxides E) water 21) 21) The element that corresponds to the electron configuration Is22s22p63s23p64s13d5 is A) titanium. B) vanadium. C) chromium. D) manganese, E) iron. 221arrow_forward
- Chrome File Edit View History Bookmarks People Tab Window Help 78% O Thu 10:02 PM A ALEKS - Jacqueline Hoppenrey x A www-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IQİHQRDYV_6Ux63SypJXz0Coxvwqgg4JkWI7XM99oekXTcojkLT31OZqp4772m0XOsdozQ5qMD3aRdEyOGQe33sgOsaJ. O GASES Calculating partial pressure in a gas mixture Jacqueline A 5.00 L tank at 7.37 °C is filled with 18.2 g of chlorine pentafluoride gas and 3.28 g of boron trifluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. Round each of your answers to 3 significant digits. 圖 mole fraction: chlorine pentafluoride partial pressure: | atm mole fraction: boron trifluoride partial pressure: | atm Total pressure in tank: | atm Explanation Check O2021 McGraw-Hill Education All Rights Reserved Terms of Use Privacy Accessibility MacBook Air 山回arrow_forwardHelp 100% 47 T. "ublic Health Ch HSC 258 - Major Projec x MindTap - Cengage Lea X C The Illustration To T =55750828934189288909969212&elSBN=9781305657571&id=D1061392007&nbld=21... * Q Search t Referonces Use the References to access important values if needed for this question. For the following reaction, 50.4 grams of sulfur dioxide are allowed to react with 17.9 grams of water. sulfur dioxide (g) + water (I) sulfurous acid (H2SO3) (g) grams What is the maximum amount of sulfurous acid (H,SO3) that can be formed? What is the FORMULA for the limiting reagent? grams What amount of the excess reagent remains after the reaction is complete? Submit Answerarrow_forwardShop Trendy CHEM 1032Cengage S19 34 https://ng.cengage.com/static/nb/ui/evo/index.html?elSBN-9781305657571 &id-430934266&isnapshotld-10652508 s19 3.4 Cengage MInbox (112) DTAP Q Search this course L ions Use the References to access important values if needed for this question. The rate constant of the elementary reaction H2(g) +Br2(g) 2HBr(g) is k 4.45x10 L mol s at 250°C, and the reaction has an activation energy of 170 kJ mol (a) Compute the rate constant of the reaction at a temperature of 289°C L mol , 1 (b) After e (b) After equal concentrations of H, and Bry are mixed at 250o C, 6,03 10' s is required for half of them to be consumed. How long will itke to consume half of the reactants if an identical experiment is performed at 289°C? Submit Answer 3 question attempts remaining Autosaved at 9:31 AM Back 9:31 2/28arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY