
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

Transcribed Image Text:ⒸMacmillan Learning
Combining 0.233 mol Fe₂O3 with excess carbon produced 13.5 g Fe.
Fe₂O3 + 3C
2Fe + 3 CO
What is the actual yield of iron in moles?
actual yield:
What is the theoretical yield of iron in moles?
theoretical yield:
What is the percent yield?
percent yield:
Expert Solution
arrow_forward
Step 1
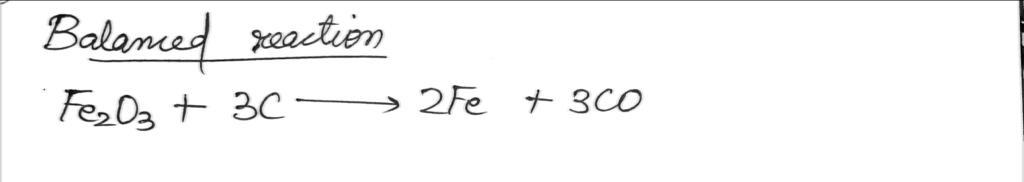
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How many moles of phosphine (PH3) are produced for every 4.0 moles of hydrogen that react according to the chemical equation below? 3H2 + P2 - 2PH3 O 4.7 moles None of the above 1.7 moles O 3.7 moles O 2.7 molesarrow_forward36)The theoretical yield of the following reaction is 43 g of NO. What is the percent yield if 28.4 g of NO are actually produced in the lab? reaction 4 NH3+ 5 O2 =4 NO+ 6 H2Oarrow_forwardQUESTION 23 Which of the following statements is false? The limiting reactant is completely consumed in a chemical reaction. O The theoretical yield is the amount of product that can be made based on the amount of limiting reagent. O The actual yield is the amount of product actually produced by a chemical reaction. O The percent yield = (actual yield/theoretical yield) x 100% O All of the above are true statements.arrow_forward
- ITS A CHEMISTRY QUESTION I NEED HELParrow_forwardWhen the following equation is balanced, the coefficient of O2 is __________. C4H8O(g) + O2 (g) → CO2(g) + H2O(g) 12 11 8 6 10arrow_forwardQuestion 86 of 97 According to the balanced reaction below, calculate the moles of NH; gas that form when 4.2 mol of N2H. liquid completely reacts: 3 N2H.(0)→ 4 NH3(g) + N2(g) STARTING AMOUNT ADD FACTOR ANSWER RESET *( ) 4.2 28.02 5.6 17.04 16.8 3.2 32.06 6.022 x 10 mol NH3 mol N2H4 g N2 g NH3 g N2H4 mol N2 MacBook Airarrow_forward
- stion O Macmillan Learning Combining 0.384 mol Fe₂O3 with excess carbon produced 16.5 g Fe. Fe₂O3 + 3C 2 Fe + 3 CO What is the actual yield of iron in moles? actual yield: What is the theoretical yield of iron in moles? theoretical yield: What is the percent yield? percent yield: Search or type URL 1 . 6 MacBook Pro & r 7 * 8 + ( 9 ) 0 w + II mol mol %arrow_forwardQuestion 4 Consider the unbalanced reaction NiS₂(s) + O₂(g) --> NIO(s) + SO₂(g) When 6.41 g of NiS₂ react with 9.37 g of O₂, 2.17 g of NiO are obtained. The theoretical yield of NiO is (number and unit) A/ the limiting reactant is (name or symbol) and the percent yield is (number) A/ %. (Remember to use correct sig figs)arrow_forwardLithium and nitrogen react to produce lithium nitride:6 Li(s) + N2(g) → 2 Li3N(s)How many moles of lithium nitride are produced when 24.0 moles of lithium react with excess nitrogen? 8.00 mol 12.0 mol 24.0 mol 48.0 mol 4.25 molarrow_forward
- Question 33 In a chemical equation, AsF3 + Cl4 - AsCl3 + CCI2F2, the theoretical yield of CCI2F2 was calculated to be 203 g. If 151 g of CCI2F2 product was obtained, what was the percent yield of this reaction? O 15.1% O 74.4% O 0.744% O 20.3%arrow_forwardHelparrow_forwardQuestion 55 pleasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY