
Chemistry: Principles and Practice
3rd Edition
ISBN: 9780534420123
Author: Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
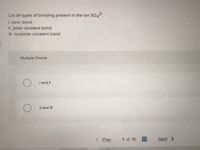
Transcribed Image Text:List all types of bonding present in the ion SO42.
1. ionic bond
II. polar covalent bond
II. nonpolar covalent bond
Multiple Choice
I and II
Il and II
K Prev
1 of 10
Next >
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the following flat drawing of methane (CH4) . a. What is HCH bond angle implied by this drawing if you assume it is flat? b. Are the electron domains of this flat CH4 spread out as much as possible? c. Use model materials to make a model of CH4 (methane). If you assembled it correctly, thefour bonds (bonding electron domains) of your model will be 109.5° apart. d. In which representation, the drawing above or the model in your hand (circle one) are theH’s of CH4 more spread out around the central carbon? e. Confirm that your model looks like the following drawing. The wedgebond represents a bond coming out of the page, and the dash bondrepresents a bond going into the page f. You will often see methane drawn as if it were flat (like on the previous page). Why is thismisleading, and what is left to the viewer’s imagination when looking at such a drawing?arrow_forward-ks Profiles Tab Window inal Pap X Workshop X PB 11.3 Pers: X D2L Homepag X ignment/takeCovalentActivity.do?locator=assignment-take A M kshop (1).docx - 0 0 = Al Help Use the References to access important values if needed for this question. From the Lewis structures of the species given, pick all of those in which the central atom obeys the octet rule. :CI: :CI-Si-CI: :CI: :C: I :Xe 1° :CI: None of the Above Submit Answer Retry Entire Group Workshop.docx NOV 28 tv (9 Akon - Be X 9 more group attempts remaining alı 8 A C From the X e CA OWLv2 | X 0 O QO Mon Now My Home X + W Previous Next> * 0 Show All Xarrow_forwardKCH12 HW ment/takeCovalentActivity.do?locator-assignment-take er Shimeji Browser Ext... C Crunchyroll - Watch... Ch 8 Page 4 ~ S-M... Backyard Origin Odownload - Photo -... 00 0 0 :0: From the Lewis structures of the species given, pick all of those in which the central atom obeys the octet rule. -Be-Br: :CI: :C-p-C: CLACI O None of the Above An error has been detected in your answer. Check for typos, miscalculations etc. before submitting your answer. Submit Answer OWLv2 | Online teaching and lea X Retry Entire Group GFrom the Lewis structures of the 9 more group attempts remaining X Lab 7: Ma Treaty of Versailles Use the Rearrow_forward
- The bond dissociation energies for the species NO, CF¯, and CF+ are ordered as CF+ > NO > CF¯. Use MO the- ory to explain this ordering.arrow_forwardwww-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IB-3LP6uMdqS-27lPnKKRzw Apps M Gmail YouTube Maps O REPRESENTATIONS OF ORGANIC MOLECULES Counting sigma and pi bonds in a small molecule Answer the questions below about the highlighted atom in this Lewis structure: :0: Н— С - Н In how many sigma bonds does the highlighted atom participate? In how many pi bonds does the highlighted atom participate? What is the orbital hybridization of the highlighted atom? Explanation Check 2 2021 P Type here to search Esc EnLk F1 F2 F3 F4 F5 F6 F7 F8 F9 23 24 4 7 8. W R T Tabarrow_forwardIdentify the correct Lewis dot structure for [CH,NH,J*. Η Η + .. H:C:N:H Η Η Η Η .. .. + H:C:N:Η HH .. Η Η + H.C:N H Η Η HH Η Η H·C:N H Ĥ Ĥ Η Ηarrow_forward
- The dipole moment (u) of HBr (a polar covalent molecule) is 0.824D (debye), and its percent ionic character is 12.2 %. Estimate the bond length of the H-Br bond in picometers. Note that . 1 D 3.34 x 10-30 Cm and in a bond with 100% ionic character, Q=1.6 x 10-19 C. Express your answer to two significant figures and include the appropriate units. View Available Hint(s) r = μA Value 6 O Units ? Inits input for part Barrow_forwardI need help on number 15.arrow_forwardHow can we predict the ideal bonds ?arrow_forward
- S Gmail Dimensional Analysis - Ak X determine the quantity of xb Answered: Ch 5 and 6 che x CA Lewis structure for L-is x how to add a formal chars x + ✰ app.aktiv.com 西☆ Tp 1 K Relaunch to update YouTube Maps Netflix in other Co... https://lms.naz.ed... Rochester to Seat... All Bookmarks STARTING AMOUNT X + ☑ 23 Question 29 of 40 Determine the quantity of oxygen atoms in 15.0 grams of Pb(NO2)2 ADD FACTOR x ( ) = ANSWER RESET D 207.20 32.00 6.022 × 1023 6.04 × 1022 299.22 15.0 2 46.01 1 16.00 4 14.01 1.21 x 1023 mol Pb mol N atoms N atoms O mol O molecules Pb(NO2)2 g Pb(NO2)2 molecules NO₂ g/mol Pb(NO2)2 mol Pb(NO2)2 atoms Pb 98,304 19 17 JUN 14 tv 80 A 0 All 2 A X ท W Submit KParrow_forwardVSEPR: Molecular Geometry 1. For the following molecules: 1) draw a Lewis Dot structure, including contributing resonance structures, if present; 2) use VSEPR to draw an appropriate structure; 3) give an appropriate designation for the ideal molecular geometry; 4) describe any deviations to the ideal molecular geometry; *don't worry about corrections for e and h. g. HOCI h.* SO;2 i. NO2*arrow_forwardThe Lewis structures of four compounds are given. H + -ci: Which of these molecules are polar? CO₂ PC13 CH₂Cl2 SO₂ -15: -P. -çi: :CI:arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning