
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
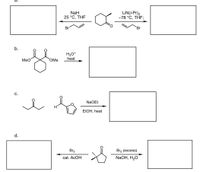
Transcribed Image Text:LiN(-Pr)2
-78°С, THF;
NaH
25 °С, THF
Br
'Br
b.
H3O*
heat
MeO
OMe
С.
NaOEt
ELOH, heat
d.
Br2
Brz (еxcess)
cat. ACOH
NaOH, H2O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1) lead + water Note: lead will be lead(II) if there is a reaction a) Pb(s) + H2O(l) ⟶⟶ No Reaction b) Pb(s) + H2O(l) ⟶⟶ PbOH(aq) + H2(g) c) Pb(s) + H2O(l) ⟶⟶ Pb(OH)2(aq) + H2(g) d) Pb(s) + 2 H2O(l) ⟶⟶ Pb(OH)2(aq) + H2(g) 2) barium + water a) Ba(s) + 2 H2O(l) ⟶⟶ H2(g) + Ba(OH)2(aq) b) Ba(s) + H2O(aq) ⟶⟶ H2(g) + Ba(OH)2(aq) c) 2 Ba(s) + 2 H2O(l) ⟶⟶ H2(g) + Ba2OH(aq) d) 2 Ba(s) + H2O(l) ⟶⟶ H2(g) + Ba2OH(aq) e) Ba(s) + H2O(l) ⟶⟶ No Reactionarrow_forwardFor the reaction below: H O". CI. H-CI a. Estimate the gas phase enthalpy change using bond dissociation enthalpies from the OWL Table Reference, not data from your text. Click the References button and then click the Tables link on the drop-down that appears. Include algebraic sign and units. b. Is the reaction exothermic or endothermic? Ⓒ c. Is the reaction likely to proceed spontaneously in the direction written?arrow_forward2. What What is the ooter of a reaction which has the kade law equalio , hate =KLAJ B]"?arrow_forward
- Solve it please...arrow_forwardWhich ions are formed when Ca(OH)2 dissolves in water to form ions? Ca2- and 2 OH+ Ca, 2-, and 2 OH, + Ca+ and OH- Ca, +, and OH, - Ca2+ O2- and H+ Ca, 2+, O, 2-, and H, + Ca2+ and 2 OH- Ca, 2+, and 2 OH, -arrow_forward30. The process for preparing dibromoethane is given in the reaction equation below. Using the bond energies listed, determine the enthalpy change (AH) for this reaction. H H нн + Brz(2) Br-С - С-Br e AH = ? kJ c=C@ нн нн Bond Bond Energy (kJ/mol) С-Н 411 C=C 602 Br-Br 190 С-Br 285 C-C 346 | Select ) [ Select + 36 kJ -124 kJ + 124 kJ + 78 kJ - 78 kJarrow_forward
- I. AHxn for the reaction below was determined using bond dissociation energies (BDES) to be -57 kJ. Using the table of BDE below, find the bond energy for H-Br. H H H Br HBr + H-C-C-H H H Bond С-С C=C C-H С-Br BDE (kJ/mol) 348 614 413 276arrow_forwardPCB, CO, HCO3, H&PO4 2.Calculate the enthalpy change for the reaction H2+ CH20 CH3OH given the following bond enthalpies: C-H=413 kJ/m, C-C = 348 kJ/m, C-0 = 358 kJ/m, C-0=743 kJ/m, H-H=436 kJ/m, O-H=463 kJ/m %3D %3D 11 %3D %3Darrow_forward(i) NBS, MeOH ? (ii) Na OEt, ELOH OMe O A. ÓEt OEt O B. ÔMe Br OC. OMe OD. Br OMearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY