
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
fill in the blank. first box is completed for you.
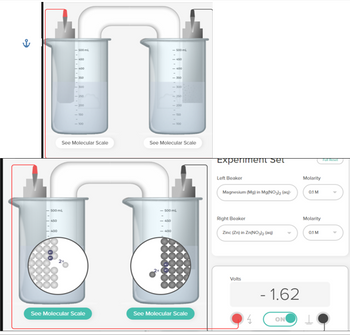
Transcribed Image Text:500 ml
400
-500 ml
See Molecular Scale
See Molecular Scale
500mL
See Molecular Scale
-500 m
See Molecular Scale
Experiment set
Left Beaker
Magnesium (Mg) in Mg(NO₂)₂ (aq)
Right Beaker
Zinc (Zn) in Zn(NO3)₂(09)
Volts
Molarity
01 M
Molarity
0.1 M
- 1.62
4 ON L

Transcribed Image Text:LEFT RIGHT
silver
(Ag)
magnesium
copper
(Cu)
zinc
(Zn)
VOLTAGE
+0.43
-1.62
MACROSCOPIC
OBSERVATIONS
Purple right-side solution and
metal warn; left side metal
has deposits.
Right side metal warns
away; left side has
deposits.
MICROSCOPIC
OBSERVATIONS
Left side electrons flow downward, attracting
additional Ag atoms to join the surface, while right
side electrons travel upward, ionizing and
dispersing the metal atoms into the solution.
LEFT SIDE
INFERENCES
cathode, reduction,
Ag+1 +1e--> Ag0
oxidation, anode,
Mg0 --> Mg+2
+20-
RIGHT SIDE
INFERENCES
anode, oxidation,
Cuo-->
Cu+2+2e-
reduction,
cathode, Zn2+
+2e---> Zn
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. C12H24(I) + O2(g)arrow_forwardinto the book. Write a concise definition of each, using examples as appropriate.arrow_forwardIn the following sub-questions, use your book and notes to generate an argument for each model of light. Remember, on the first day of class we discussed that argumentation requires: ● a claim (which I've given), • evidence (which you should look up in the form of data or scientific principles) and reasoning that connects the evidence to the claim (which you should generate from your understanding so far).arrow_forward
- View History Bookmarks Develop Window Help A east.cengagenow.com [References) 1 pt EXERCISE Unit Conversion 1 pt Drag conversion units onto the boxes in the equation to make conversions. Some boxes can be left empty. Click on a unit to remove it from its position. 1 pt 1 cm³ Cu 9 g Cu 9.5 x 1021 atoms Cu 1 g Cu 1 pt 1 kg 1000 g 1 cm = 1 mL 1 pt 1 L 1000 cm3 1 pt 1 pt 520 L x 1 pt 1 pt 1 pt A piece of copper has a volume 520 L. What is the mass of the sample, in units of grams? In the boxes above, enter the correct setup that would be used to solve this problem. 1 pt 1 pt Check Next (2 of 3) 1 pt 1 pt Submit Answer Try Another Version 2 item attempts remaining 1 ptarrow_forwardC. The lead(II) oxide was weighed before and after the additions. d. 19 18 17 16 9 before لسلا ii. What method is used to separate the mixture in stage 3? 15 iii. What term is used to describe the unreacted lead(II) oxide? 14 13 12 9 Use the balance diagrams to work out the mass of lead(II) oxide added to the dilute nitric acid. after i. How would the student know when all of the dilute nitric acid had reacted in stage 2? e. Describe the effect of heating the solution of lead(II) nitrate until it boils and then heating for a further ten minutes.arrow_forwardRank the following diatomic species of fluorine in order of bond length and bond strength. A. F2 B. F₂+ C. F₂ Bond Length Bond Strength Longest Next Shortest Strongest Next Weakestarrow_forward
- Please answer question number 1 correctly with the correct answer. there is a picture of an example of a ''Dimensional analysis Format' below. please write out all work showing the math in dimensional analysis format. Question 3: Calculate the molar mass of each of the following ionic Compounds: A. KMnO4 B. Ca3(PO4)2arrow_forwardPart A Identify each of the labeled points (indicated with letters) or changes (indicated with two letters separated by an arrow) shown on the phase diagram. 1.00- Pressure (atm) 0.50- A 0.10- F -150 -75 75 150 Temperature ("C)arrow_forwardI am struggling to get this question right and would really appreciate any help. Pls make sure you’re anser is corrrecf 100% pls and thank you !arrow_forward
- Unit 3-Two slides 11. Create a question that involves your compound and the formula m= n.M. Solve the question and show all of your work. wwwww 12. Create a question that involves your compound and the formula n = N/NA. Solve the question and show all of your work. TEarrow_forwardWhat is calibration and why is it essential in relation to food analysis? Provide examples.arrow_forwardinto the book. Write a concise definition of each, using examples as appropriate.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY