
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Electrolytic Reaction. 1) Is it Conrotatory or Disrotatory? 2) Thermal or Photochemical? 3) How many Electron pairs Involved?

Expert Solution
arrow_forward
Step 1
Given reaction is
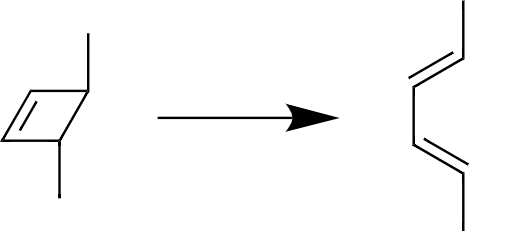
To explain
1) whether the reaction is Conrotatory or Disrotatory
2) whether the reaction is Thermal or Photochemical
3) How many Electron pairs Involved in this reaction.
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Why can’t the ∆H for the reaction of Mg with water be measured directly?arrow_forwardNNO(g) Incorrect : Narrow_forwardWhich are important thermodynamic parameters associated with the completion of a single step reaction? Explain its energy profile diagram. Sketch diagrams for exo-and endothermic reactions?arrow_forward
- Please help me with question 19.67.arrow_forwardFor each of the following sets of data, identify the entity that theoretically would produce those results. Strongly conducts electricity and turns red litmus blue. Weak conductor of electricity and slowly reacts with Mg(s) Weakly conducts electricity and does not react at all with Mg(s) Tastes bitter and strongly conducts electricity HNO₂ HCI HOCI NaCl HI NH3 KOH H₂CO3 NaCN LIOH Na₂CO3 HI KOH H₂SO3 CH3OH NaOHarrow_forwardPlease don't provide handwritten solution ....arrow_forward
- [Review Topics] [References] Use the References to access important values if needed for this question. For the reaction 2Na(s) + 2H₂O(1)→ 2NaOH(aq) + H₂(g) AH = -368.6 kJ and AS° = - 15.3 J/K The standard free energy change for the reaction of 1.87 moles of Na(s) at 300 K, 1 atm would be kJ. This reaction is standard conditions at 300 K. Assume that AH and AS are independent of temperature. favored under Submit Answer 9 more group attempts remaining Retry Entire Group Nextarrow_forwardHow many of the processes below have a positive AS? H;O() - H;O(g) The heating of 35 g of water from 99°C to 100°C Cu()- Cu(s) 2 NH(g) - Nalg) + 3 Ha(g) OAO 084 OC1 OD.2 OE3arrow_forward1.) For the following reaction [Cd(H₂O),]+2(aq) + Br a.) If AgNO3(aq) is added what direction would the reaction (aq) → [Cd(H₂O),Br]*(aq) + H₂O() shift to and why? Note that: Ag (aq) + Br (aq) → AgBr(s)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY