
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
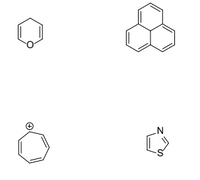
Label each as aromatic, antiaromatic, or nonaromatic
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the main difference between an aromatic and an anti-aromatic compound? O None of these O Aromatic compounds must satisfy Hückel's rule. O Anti-aromatic compounds must have a conjugated system with a p orbital at every vertex. O Aromatic compounds must be cyclic and planar but not anti-aromatic compounds. O Aromatic compounds must be monocyclic.arrow_forwardWhat is the molecular formula (C before H) for methylbut ane?arrow_forwardPart C Spell out the full name of the compound. 1-4-dimethylcyclohex-1-ene Submit Previous Answers Request Answer X Incorrect; Try Again; 3 attempts remaining Part D CI CI Spell out the full name of the compound. 4-6-dimethylhept-1-ene Submit Previous Answers Request Answer X Incorrect; Try Again; 6 attempts remainingarrow_forward
- Nonearrow_forwardIs the following compound aromatic, antiaromatic, or nonaromatic?arrow_forwardClassifying a carbon atom by the number of carbons to which it is bonded can also be done in more complex molecules that contain heteroatoms. Classify each sp' hybridized carbon atom in bilobalide, a compound isolated from Ginkgo biloba extracts, as 1°, 2°, 3°, or 4°. Be sure to answer all parts. b НО -h a O: bilobalide а: b: c: d: e: f: g: Oarrow_forward
- Iupac name? Need answer step by steparrow_forwardis this molecule aromatic answer by drawing orbitals (VBT), applying huckels law, and drawing frosts circlearrow_forwardC. D. O: :O: The lone pair in compound C is Compound C is In compound D, not aromatic. aromatic. delocalized. not delocalized. one lone pair is delocalized. both lone pairs are not delocalized. both lone pairs are delocalized. Compoundarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY