
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
A flask is charged with 1.750 atmatm of N2O4(g)N2O4(g) and 1.00 atmatm of NO2(g)NO2(g) at 25 ∘C∘C , and the following equilibrium is achieved:
N2O4(g)⇌2NO2(g)N2O4(g)⇌2NO2(g)
After equilibrium is reached, the partial pressure of NO2 is 0.513 atmatm .
A. What is the partial pressure of N2O4N2O4 at equilibrium?
Express the pressure to three significant figures and include the appropriate units.
B.Calculate the value of KpKp for the reaction.
Express your answer to three significant figures.
Expert Solution
arrow_forward
Step 1
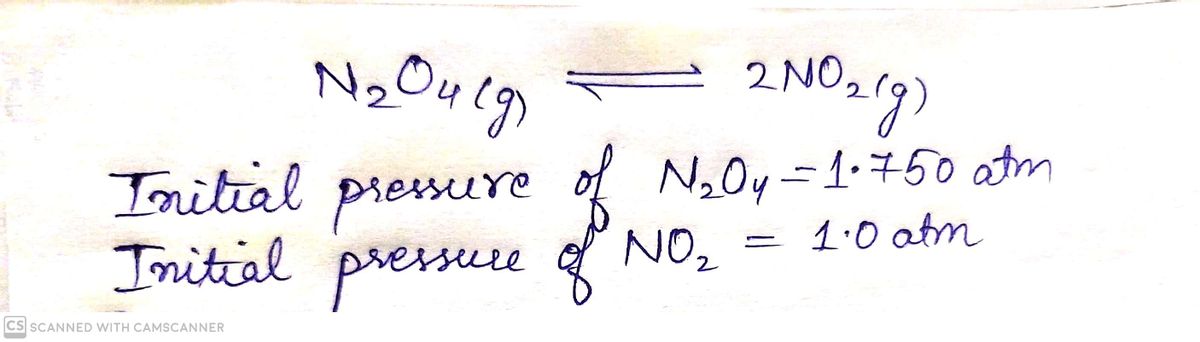
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the following reaction H₂(g) + I₂(g) ⇌ 2HI(g) ∆H° = -10.64 kJ/mol and ∆S° = 21.48 J/mol・K. A closed container in which P_H₂ = P_I₂ = 0.100 atm and P_HI = 0.500 atm is heated to 655 °C at constant pressure. After equilibrium is attained at that temperature, what is the pressure of HI in atm? Assume ∆H° and ∆S° are temperature independent. Start by calculating ∆G° and then find Kp. Then use an ICE table.arrow_forwardHydrogen sulfide decomposes according to the following reaction, for whichKc = 9.30 × 10−8 at 700°C:2 H2S(g) ⇌ 2 H2(g) + S2(g)If 0.31 mol of H2S is placed in a 3.0−L container, what is the equilibrium concentration of H2(g) at 700°C?Marrow_forwardNitrogen and oxygen react at high temperatures. For the reversible reaction, N2(g) + O2(g) ⇌ 2NO(g) ΔH=181kJ What will happen to the concentrations of N2, O2, and NO at equilibrium after the temperature of the system is increased? What will happen to the concentrations of N2, O2, and NO at equilibrium if a catalyst is added?arrow_forward
- Consider the reaction: N₂ (9) +202 (g) ⇒ N₂O4 (9) Write the equilibrium constant for this reaction in terms of the equilibrium constants, K₁ and K₂, for the reactions below: N₂O4 (9) 2NO₂(g) K₁ 1/2N₂(g) + O₂(g) NO₂ (9) K₂ For answers with both a subscript and a superscript, enter the subscript first. For example, enter Kif the first equilibrium constant should be squared. K =arrow_forwardNitrogen and water react to form nitrogen monoxide and hydrogen, like this: N2(9) + 2 H,O(g) 2 NO(g) + 2 H2(g) → Also, a chemist finds that at a certain temperature the equilibrium mixture of nitrogen, water, nitrogen monoxide, and hydrogen has the following composition: compound pressure at equilibrium N2 92.2 atm H,O 76.1 atm NO 19.3 atm H2 33.6 atm Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits. K = 0arrow_forwardWrite the Kc and Kp (if applicable) expressions for the following heterogeneous equilibrium reactions: a)2Hg(l)+O2(g)⇌2HgO(s) b)C(s)+2H2(g)⇌CH4(g) c)2Mg(s)+O2(g)⇌2MgO(s)arrow_forward
- What is the value of the equilibrium constant for the reaction below if the partial pressures at equilibrium are [PH3] = 0.022 atm, [P2] = 0.289 atm, and [H2] = 0.867 atm at 873 K? 2 PH3 (g) ⇌ P2 (g) + 3 H2 (g)arrow_forwardAs shown in Table 15.2 in the textbook, KpKp for the equilibriumN2(g)+3H2(g)⇌2NH3(g)N2(g)+3H2(g)⇌2NH3(g)is 4.51×10−54.51×10−5 at 450 ∘C∘C. For each of the mixtures listed here, indicate whether the mixture is at equilibrium at 450 ∘C∘C. If it is not at equilibrium, indicate the direction (toward product or toward reactants) in which the mixture must shift to achieve equilibrium. A. 98 atmatm NH3NH3, 45 atmatm N2N2, 55 atmatm H2H2 98 , 45 , 55 a. yes, mixture is at equilibrium b. no, mixture must shift toward products to achieve equilibrium c. no, mixture must shift toward reactants to achieve equilibrium B.57 atmatm NH3NH3, 143 atmatm N2N2, no H2H2 57 , 143 , no a.yes, mixture is at equilibrium b.no, mixture must shift toward products to achieve equilibrium c.no, mixture must shift toward reactants to achieve equilibriumarrow_forwardHydrogen is prepared commercially by the reaction of methane and water vapor at elevated temperatures. CH4(g)+H2O(g)⇌3H2(g)+CO(g) What is the equilibrium constant for the reaction if a mixture at equilibrium contains gases with the following concentrations: CH4, 0.126 M; H2O, 0.242 M; CO, 0.126 M; H2 1.15 M, at a temperature of 760. °C? Enter your answer with 3 sig figs.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY