
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
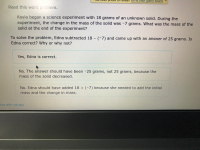
Transcribed Image Text:Kayla began a science experiment with 18 grams of an unknown solid. During the
experiment, the change in the mass of the solid was -7 grams. What was the mass of the
solid at the end of the experiment?
To solve the problem, Edna subtracted 18 - (-7) and came up with an answer of 25 grams. Is
Edna correct? Why or why not?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- CL=( A-B ) *N * 35450/Volume of sample, N is ? Select one: a. N is the volume of the sample b. N is Neutralization c. N is Natural water d. N is Normalityarrow_forwardWho wrote it Charles Dickens/Patricia Cornwell. 2 It's a family saga/It's a thriller/It's a biography. 3 ? It's about a tragic family/It's about the murder of a detective. 4 Where and In India in the 19th century/In New York in the 1990s. 5 A lawyer called Potts and his client, Lady Jane /A detective called Blunket. 6 Yes, it has. It came out a few years ago and starred Johnny Depp. 7 It ends really tragically/It's frustrating because we don't really know/They all live happily ever after. 8 ? I thought it was great/I couldn't put it down/I didn't want it to end/It was OK but I skipped the boring bits. 9 ? Yes, I would. It's great if you like a good thriller/It's a terrific holiday read.arrow_forwardWhat is the volume of 1000 moles of methanol at 298 K and 20.0 bar? (Answer in m³ and use at least three significant digits.) Selected Answer: 1223.29 Correct Answer: 0.0409 Answer range +/- 0.00005 (0.040850 - 0.040950)arrow_forward
- If u dont know please suggest to other Experts. Asap solve all Handwritingarrow_forwardCalculate the Biot Number to determine if the Lumped Parameter Analysis can be used. If the Biot Number is greater than 0.10, use the Temperature-Time charts in Appendix F to calculate an answer and compare the result to the answer based on lumped capacitance. appendix F in this textbook https://drive.google.com/file/d/145Cbs8i465qUq9JhBFhia58fnPkuhU3k/view?usp=sharingarrow_forwardCan you do this pleasearrow_forward
- Can you help me pleasearrow_forwardPart I How much energy is required to ionize (remove the electron from) the hydrogen atom in the n = 3, l= 1 state? Answer in units of electron volts to four significant figures. Do not include the units symnbol. ΑΣφ eV Submit Request Answerarrow_forwardA metal has density of 19.58 g/cm3, fcc structure and atomic radius of 0.15 nm. Calculate the approximate atomic weight? This question has only one correct answer. Question 10Answer a. atomic weight =253.3 b. atomic weight =225.2 c. atomic weight =197.0 d. atomic weight =112.6 % e. atomic weight =323.7arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The