
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
This is a titration acid base problem. The material list is for Part A question 1. Thank you!
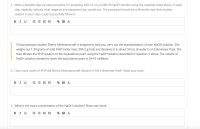
Transcribed Image Text:1. Write a detailed step-by-step procedure for preparing 100 mL of a 0.200 M NaOH solution using the materials listed above. In each
step, explicitly indicate what reagents and equipment you would use. The procedure should be sufficiently clear that another
student in your class could successfully follow it.

Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 5)I need part carrow_forwardS NGSS CHEMISTRY GT S Schoology O Paraphrasing Tool | Qu W Word Doc A bcps.schoology.com/common-assessment-delivery/start/4979553967?action3Donresume&submissionid O BCPS Links 9 Home | Schoology Word 6 Original NBA strea. O Driver Education, O. Show What You Know: pH & pOH What is the pH of a solution with [OH] = 6.9 x 10-2 M? Show your work.arrow_forwardThank you for the assistance! really appreciate it!arrow_forward
- Assuming equal concentrations, arrange these solutions by pH. Highest pH HNO, (aq) AICI, (aq) CaBr, (aq) LIOH(aq) K,CO, (aq) Lowest pH Answer Bankarrow_forwardA.) The pH of an aqueous solution at 25°C was found to be 8.30.The pOH of this solution is .The hydronium ion concentration is M.The hydroxide ion concentration is M. B.) The pOH of an aqueous solution at 25°C was found to be 7.80.The pH of this solution is .The hydronium ion concentration is M.The hydroxide ion concentration is M.arrow_forwardType your anSwers in all of the Let's consider two dilute acid solutions that are both made at a concentration of 0.010 M. One solution is made with HCl and one solution is made with HClO. Complete the following sentences based on these two solutions. HCl is a Type your answer here acid while HCIO is a Type your answer here acid. (strong, weak) The pH of the HCl solution is Type your answer here Due to the difference in acid strength, HCIO dissociates Type your answer here than HCl. (more, less) This means that there is Type your answer here (more, less) Htor H.0t produced in the HCIO solution than in the HCl solution. Higher hydronium ion concentration corresponds to a Type your answer here pH value. (higher, lower) Altogether, this means that the pH of the HCIO solution is Type your answer here than the HCl solution. (higher, Fullscreen lower) 10:40 PM a の 5/8/2021 hp enter pause ↑ shift endarrow_forward
- Q7: what is the correct answer choice REPORT ANSWER TO TWO DECIMAL PLACEarrow_forwardPlease help!!!!arrow_forwardder these chemical species by increasing pH of an 0.1 M aqueous solution of each. That is, imagine making an 0.1 M solution of each species. Select I next to the species that makes the solution with the lowest pH. Select 2 next to the species that makes the solution with the next higher pH, and so on. Notice that some of the rankings have been filled in for you already. Also notice that water is on the list. For that particular case, just compare the pH of pure water to the pH of the other solutions. Note for advanced students: for all charged species, you may assume the necessary counterions act as neither acids nor bases. relative pH of species 0.1 M aqueous solution H₂O HN3 C₂H5N 2 7 F N3 (Choose one) 1 (lowest) 234 OH C,HẠNH HF 5 6 + 7 8 (highest) 1 (lowest)arrow_forward
- A) how many miles of NaOH were required to reach the equivalence point ? B) how many moles of NaOH were required to reach the equivalence point ? C) How many moles of CH3COOH were present in the initial sample of acid ? D) what was the exact concentration of the initial acetic solution ?arrow_forward2. The pH of a 2.14 M solution of a weak base Bis measured to be 10.82 Determine K, of the base Determine K, of the weak bases's conjugate acid, HB* Determine [H*] in a 2.00 M solution of the chloride salt, HBCI Determine the pH of a 2.00 M solution of the chloride salt, HBCIarrow_forwardSuppose a 0.14 M aqueous solution of oxalic acid (H₂C₂O₂) is prepared. Calculate the equilibrium molarity of CO,. You'll find information on the properties. of oxalic acid in the ALEKS Data resource. Round your answer to 2 significant digits. M D.P X Sarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY