
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
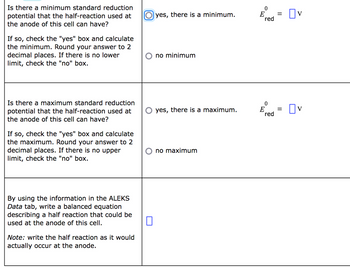
Transcribed Image Text:Is there a minimum standard reduction
potential that the half-reaction used at
the anode of this cell can have?
If so, check the "yes" box and calculate
the minimum. Round your answer to 2
decimal places. If there is no lower
limit, check the "no" box.
Is there a maximum standard reduction
potential that the half-reaction used at
the anode of this cell can have?
If so, check the "yes" box and calculate
the maximum. Round your answer to 2
decimal places. If there is no upper
limit, check the "no" box.
By using the information in the ALEKS
Data tab, write a balanced equation
describing a half reaction that could be
used at the anode of this cell.
Note: write the half reaction as it would
actually occur at the anode.
Oyes, there is a minimum.
no minimum
yes, there is a maximum.
no maximum
0
E 0V
red
0
E
red
=
=
☐v

Transcribed Image Text:A certain half-reaction has a standard reduction potential E -0.89 V. An engineer proposes using this half-reaction at the cathode of a galvanic cell that
red
must provide at least 0.70 V of electrical power. The cell will operate under standard conditions.
Note for advanced students: assume the engineer requires this half-reaction to happen at the cathode of the cell.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A chemist designs a galvanic cell that uses these two half-reactions: half-reaction 2 IO3(aq)+12 H*(aq)+10e¯ 1₂(s) + 6H₂O(1) 2+ Cu²+ (aq)+e Cu (aq) Answer the following questions about this cell. Write a balanced equation for the half-reaction that happens at the cathode. Write a balanced equation for the half-reaction that happens at the anode. Write a balanced equation for the overall reaction that powers the cell. Be sure the reaction is spontaneous as written. Do you have enough information to calculate the cell Yes 1 standard reduction potential = +1.195 V Ered red = +0.153 Varrow_forwardA chemist designs a galvanic cell that uses these two half-reactions: half-reaction standard reduction potential 2 H,O(1)+2e H,(9)+2ОН (аq) E red -0.83 V Cu"(aq)+e 2+ - Cu (aq) End=+0.153 V red Answer the following questions about this cell. Write a balanced equation for the half-reaction that happens at the cathode. Write a balanced equation for the half-reaction that happens at the anode. Write a balanced equation for the overall reaction that powers the cell. Be sure the reaction is spontaneous as written.arrow_forwardWrite a balanced equation for the half-reaction that happens at the cathode. Write a balanced equation for the half-reaction that happens at the anode. 中 Write a balanced equation for the overall reaction that powers the cell. Be sure the reaction is spontaneous as written. Do you have enough information to calculate the cell voltage under standard conditions? O О If you said it was possible to calculate the cell voltage, do so and enter your answer here. Round your answer to 2 significant digits. Ον No Yes ローロ e ☐ x10 Garrow_forward
- 0 A certain half-reaction has a standard reduction potential E 'red must provide at least 0.70 V of electrical power. The cell will operate under standard conditions. Note for advanced students: assume the engineer requires this half-reaction to happen at the anode of the cell. Is there a minimum standard reduction potential that the half-reaction used at the cathode of this cell can have? If so, check the "yes" box and calculate the minimum. Round your answer to 2 decimal places. If there is no lower limit, check the "no" box. Is there a maximum standard reduction potential that the half-reaction used at the cathode of this cell can have? If so, check the "yes" box and calculate the maximum. Round your answer to 2 decimal places. If there is no upper limit, check the "no" box. By using the information in the ALEKS Data tab, write a balanced equation describing a half reaction that could be used at the cathode of this cell. Note: write the half reaction as it would actually occur at…arrow_forwardZinc is used as a “sacrificial” anode on hulls of salt water vessels made of iron. The effectis to reverse the oxidation of iron to iron (II) by causing zinc to oxidize to the zinc ion. Calculate the voltage for this reaction if it takes place under standard conditions.arrow_forwardHelp with the following question The 2nd photo is a close uparrow_forward
- The answer is supposed to be in gramsarrow_forwardby: Th The concentration cell to the left was allowed to operate until the cell reaction reached equilibrium. What are the cell potential and the concentrations of zinc(II) in each half-cell for the cell now?arrow_forwardUse smallest possible integer coefficients. If a box is not needed, leave it blank. Use a table of Standard Reduction Potentials to predict if a reaction will occur between Hg metal and I,(s), when the two are brought in contact via standard half-cells in a voltaic cell. If a reaction will occur, write a balanced net ionic equation for the reaction, assuming that the product ions are in aqueous solution. If no reaction will occur, leave all boxes blank.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY