
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
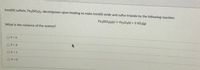
Transcribed Image Text:Iron(III) sulfate, Fe2(SO4)3, decomposes upon heating to make iron(III) oxide and sulfur trioxide by the following reaction:
Fe2(SO4)3{s) = Fe,Og(s) + 3 SO3(g)
What is the variance of the system?
OF= 2
OF= 3
OF=1
OF= 0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- 2. please answer these 2 for mearrow_forwardThe combustion of octane, C8H18,C8H18, proceeds according to the reaction shown. 2C8H18(l)+25O2(g)⟶16CO2(g)+18H2O(l)2C8H18(l)+25O2(g)⟶16CO2(g)+18H2O(l) If 4.10×102 mol4.10×102 mol of octane combusts, what volume of carbon dioxide is produced at 19.0 ∘C19.0 ∘C and 0.995 atm?0.995 atm?arrow_forwardConsider the following reaction: C3H13(1) + 25/2 O»(g) → 8 CO2(g) + 9 H20(1) If d[CO2]/dt = 0.5OOM/s, determine the value of d[O2]/dt,arrow_forward
- 11. Using Hess' Law, calculate the AHOrxn for the following reaction: CH4 (8) + 1/2O2 (8) CO (8) + 2 H2 (8) The following information is given: CH4 + 202 (8) (8) CH4 + CO₂ (g) (8) CH4 + H₂O (g) (g) → → CO2 (g) + 2 H₂O (1) 2 CO (8) 2 H2 (g) + CO(g) + 3 H₂ (g) AH = ΔΗ = - 802 kJ 247 kJ ΔΗ - 206 kJarrow_forwardHw.180.arrow_forwardLauto.d21?ou=2194845&isprv3D&drc=0&qi=2824083&cfql=0&dnb=D0&f Calculate the standard enthalpy change for the reaction 2C8H18(1) + 17O2(g) → 16CO(g) + 18H20(1) Given: 2C3H18(1) + 2502(g) – 16CO2(g) + 18H20(1) AH° = -11020 kJ/mol 2C0(g) + O2(g) – 2CO2(g) AH° = -566 kJ/mol a) 10450 kJ/mol b) -6492 kJ/mol O c) 15550 kJ/mol d) 6492 kJ/mol e) -10450 kJ/molarrow_forward
- Calculate A Hrxn for the following reaction: 2C,H4 (g) + 2H2 (g) →2C2H6 (g) Using the following data and Hess's Law. C2H4 (g) + 302 (g) - 2CO2 (g) + 2H2O () ΔΗ -1411 kJ = 2C2H6 (g) + 702 (g) 4CO2 (g) + 6H2O (1) ΔΗ -1560 kJ = 2H2 (g) + O2 (g)- → 2H20 (g) AH= -285.8 kJarrow_forwardFor the states and processes shown below. Calculate 1 W3 in Joules: 3 Pi= P3=200Kta P= 50KPa 2 V3=1m?arrow_forward2HNO3 (1) + 6HB1(g) 4H2O(1) + 2NO(g) + 3B12 (1) Standard Entropies at 25°C (J/mol K) HBr(g) 198.6 Br2 (1) HNO3 (1) 152.2 155.6 NO(g) 210.7 H2O(1) 69.9 AS J/K 3C12 (g) + 3H2O(1) → 5C1¯(ag) + Cl03 (aq) + 6H* (ag) Standard Entropies at 25°C (J/mol·K) Cl2 (9) Cl03 (ag) CI (ag) H* (ag) H,O(1) 223.0 162.3 56.5 0.0 69.9 AS° = J/K 2MNO4 (ag) + 6I (ag) + 8H* (ag) 312 (s) + 2MnO2(s) + 4H2O(1) Standard Entropies at 25°C (J/mol K) I (ag) 111.3 I2 (8) 116.1 MnO4 (aq) 191.2 MnO2 (s) 53.0 H* (ag) 0.0 H2O(1) 69.9 AS° J/Karrow_forward
- Calculate the average deviation of the following specific heat determinations: 0.18 J/g °C 0.34 J/g °C 0.27 J/g °Carrow_forwardplease show me how to answer this using hess lawarrow_forward1) PCI3(1) + Cl2(g) - PCI5(g) Jse equations (2) and (3) to calculate AHrxn of equation (1): 2) P4(s) + 6Cl2(g)→ 4PCI3(1) AH =-1280 kJ 3) P4(s) + 10C12(g) → 4PCI5(g) AH =-1774 kJarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY