
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
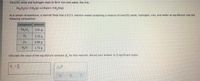
Transcribed Image Text:Iron(III) oxide and hydrogen react to form iron and water, like this:
Fe,0,(s)+3 H,(9)-2 Fe(s)+3 H,O(9)
At a certain temperature, a chemist finds that a 9.5 L reaction vessel containing a mixture of iron(III) akide, hydrogen, iron, and water at equilibrium has the
following composition:
compound amount
Fe,O,
241
H,
3.53 g
Fe
4,06 8
H,0
1.72 g
Calculate the value of the equilibrium constant K for this reaction. Round your answer to 2 significant digits.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 4arrow_forwardNitric acid and nitrogen monoxide react to form nitrogen dioxide and water, like this: 2 HNO3(aq)+NO(g)→ 3 NO,(g)+H,0(1) At a certain temperature, a chemist finds that a 4.5 L reaction vessel containing a mixture of nitric acid, nitrogen monoxide, nitrogen dioxide, and water at equilibrium has the following composition: compound amount dlo HNO3 14.2 g 18 Ar NO 7.3 g NO, 19.2 g H,O 87.0 g Calculate the value of the equilibrium constant K for this reaction. Round your answer to 2 significant digits. K = 0 x10 ?arrow_forwardSulfur dioxide can react with oxygen gas as follows:SO2(g) + ½ O2(g) SO3(g) K = 2.79 x 1012If 0.88 mol of SO2(g) and 0.25 mol of SO3(g) are placed in a 1.00 L container, calculate the final concentration of each gas at equilibriumarrow_forward
- Iron(III) oxide and hydrogen react to form iron and water, like this: Fe,O3(s)+3 H2(9)–→2 Fe(s)+3 H,O(g) At a certain temperature, a chemist finds that a 4.5 L reaction vessel containing a mixture of iron(III) oxide, hydrogen, iron, and water at equilibrium has the following composition: compound amount Fe,O3 3.54 g H2 3.20 g Fe 4.49 g H,O 1.06 g Calculate the value of the equilibrium constant K_ for this reaction. Round your answer to 2 significant digits.arrow_forwardFill in the missing reactant, reagent and products. Indicate stereochemistry if necessary. Unless otherwise specified, assume the reagents are in excess.arrow_forwardTitanium(IV) chloride decomposes to form titanium and chlorine, like this: TİCL,(1)→Ti(s)+2 Cl,(9) At a certain temperature, a chemist finds that a 3.4 L reaction vessel containing a mixture of titanium(IV) chloride, titanium, and chlorine at equilibrium has the following composition: compound amount TiCl, 3.86 g Ti 2.68 g Cl2 1.40 g Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits. K_ = ] x10arrow_forward
- When 7.121 x 10-1 mol of NH3 and 7.121 x 10-1 mol of O2 are introduced into a 2.305 L reaction vessel at a certain temperature a chemist finds that at equilibrium the N2 concentration is 1.003 x 10-1 M: 4NH3(g) + 3O2(g) <==> 2N2(g) + 6H2O (g) What is the value of Kc for this reaction ?arrow_forwardA student artificially pumped 1 mole of each substance seen below in a piston with a moveable lid (this lid can increase or decrease thereby changing the volume and pressure): 2 C3H7OH (g) + 902 (g) 6 CO2 (g) +8 H2O(g) If the student increased the volume of the piston, how will the reaction shift to reach a new equilibrium? No conclusion can be made since more information is needed The reaction will shift to the left The reaction will shift to the right The reaction will not shift because Q = Karrow_forwardCalculating an equilibrium constant from a partial equilibrium composition Ammonia has been studied as an alternative "clean" fuel for internal combustion engines, since its reaction with oxygen produces only nitrogen and water vapor, and in the liquid form it is easily transported. An industrial chemist studying this reaction fills a 500. mL flask with 3.3 atm of ammonia gas and 0.87 atm of oxygen gas, and when the mixture has come to equilibrium measures the partial pressure of water vapor to be 1.6 atm. Calculate the pressure equilibrium constant for the combustion of ammonia at the final temperature of the mixture. Round your answer to 2 significant digits. K = 0 P ☐ x1 x10 ☑ 5arrow_forward
- Consider the following reaction where Kc = 7.00 × 10-5 at 673 K. NH₂I(s) NH3(g) + HI(g) A reaction mixture was found to contain 0.0532 moles of NHI(s), 0.00571 moles of NH3(g), and 0.00837 moles of HI(g), in a 1.00 liter container. Is the reaction at equilibrium? If not, what direction must it run in order to reach equilibrium? The reaction quotient, Qc, equals The reaction must run in the forward direction to reach equilibrium. © must run in the reverse direction to reach equilibrium. O is at equilibrium.arrow_forwardNitrogen and water react to form nitrogen monoxide and hydrogen, like this: N2(9) + 2 H,O(g) 2 NO(g) + 2 H2(g) → Also, a chemist finds that at a certain temperature the equilibrium mixture of nitrogen, water, nitrogen monoxide, and hydrogen has the following composition: compound pressure at equilibrium N2 92.2 atm H,O 76.1 atm NO 19.3 atm H2 33.6 atm Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits. K = 0arrow_forwardIron and water react to form iron(III) oxide and hydrogen, like this: 2 Fe(s)+3 H,0(g)→ Fe,O3(s)+3 H,(g) At a certain temperature, a chemist finds that a 9.2 L reaction vessel containing a mixture of iron, water, iron(III) oxide, and hydrogen at equilibrium has the following composition: compound amount ol. Fe 4.85 g Ar H,O 1.44 g Fe,O3 2.98 g H2 2.51 g Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits. K_ = 0 Ox10 Explanation Check © 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY