
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
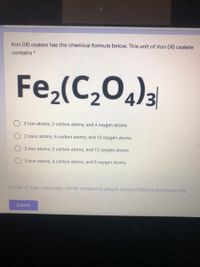
Transcribed Image Text:Iron (III) oxalate has the chemical formula below. This unit of iron (II) oxalate
contains *
Fe,(C,0)3
3 iron atoms, 2 carbon atoms, and 4 oxygen atoms
2 irons atoms, 6 carbon atoms, and 12 oxygen atoms
2 iron atoms, 2 carbon atoms, and 12 oxygen atoms
3 iron atoms, 4 carbon atoms, and 8 oxygen atoms
A copy of your responses will be emailed to abigail.santos704@stu.kipptexas.org.
Submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The element argon has three isotopes and an average atomic mass of 39.948 u. Idenitfy the most abundant isotope of element argon. argon-38 O cannot determine from the information given O argon-36 O argon-40 79°F Aarrow_forwardNumber 63arrow_forwardThe element argon has three isotopes and an average atomic mass of 39.948 u. Idenitfy the most abundant isotope of element argon. argon-38 cannot determine from the information given argon-36 argon-40 3:39 PM ch S.W 2020 79°F 9/8/2021 AV 10 DII delete f9 12 144 & L. backspace home 4. 8 pg up T. Y 心) %24 林arrow_forward
- How many protons, neutrons, and electrons?arrow_forwardFor the following elements state their Name, Period, Group #, Family Name and type of element? Name Period Group # Family Туре Element Na Fe Br Xe Caarrow_forwardFind the average atomic mass (atomic weight) for lithium on the periodic table. There are only two significant isotopes of lithium, Li-6 and Li-7. Which is the most abundant isotope ? Not a calculation.arrow_forward
- The proton and the electron have about the same mass. Most of the mass of an atom is due to The neutron is electrically neutral. the protons and neutrons. The mass number is the number of The charge of a proton is equal, but opposite, to the charge of a neutron. protons. True False More information needed to make a determinationarrow_forwardWhat is the chemical formula for the compound formed between iron(II) and chlorine? chemical formula: What is the chemical formula for the compound formed between iron(II) and selenium? chemical formula: MAR 29 图 MacBook Air 80 DII DD F3 F4 F6 F7 F8 F9 F10 # $ & 3 4 7 8 R Y U O P { F H J K C V M V * 00arrow_forward31. Which of the following parts of Dalton's theory were proven incorrect with newer versions of the atomic theory? Group of answer choices a) An element is made of atoms that are all the same size and mass. b) Atoms combine to form chemical compounds c) Chemical Comoundds are formed in whole number ratios. d) The same elements may be combined in different ratios to form different compounds.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY