
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:=
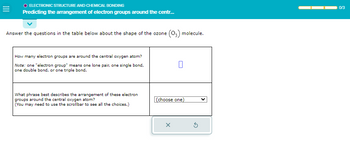
O ELECTRONIC STRUCTURE AND CHEMICAL BONDING
Predicting the arrangement of electron groups around the centr...
Answer the questions in the table below about the shape of the ozone (03) molecule.
How many electron groups are around the central oxygen atom?
Note: one "electron group" means one lone pair, one single bond,
one double bond, or one triple bond.
What phrase best describes the arrangement of these electron
groups around the central oxygen atom?
(You may need to use the scrollbar to see all the choices.)
(choose one)
X
0/3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Answer the questions in the table below about the shape of the phosphorus pentabromide (PBr,) molecule. How many electron groups are around the central phosphorus atom? Note: one "electron group" means one lone pair, one single bond, one double bond, or one triple bond. What phrase best describes the arrangement of these electron groups around the central phosphorus atom? (You may need to use the scrollbar to see all the choices.) (choose one) Ar Explanation Check © 2021 McGraw Hill LLC. AlI Rights Reserved. Terms of Use Privacy Center | Accessibilityarrow_forwardDraw Lewis structures for Ozone, O3 Number of electron sets (groups): ? Electronic Geometry: ? Number of bonding electron sets (groups): ? Number of non-bonding electron sets (groups) or lone pairs: ? Molecular Geometry: ? How many bonds have a dipole: ? If present, do the dipole cancel each other: ? Is the molecule polar: ?arrow_forward||| = 43°F Clear O ELECTRONIC STRUCTURE AND CHEMICAL BONDING Predicting the arrangement of electron groups around the centr... Answer the questions in the table below about the shape of the chlorine pentafluoride (CIF) molecule. How many electron groups are around the central chlorine atom? Note: one "electron group" means one lone pair, one single bond, one double bond, or one triple bond. What phrase best describes the arrangement of these electron groups around the central chlorine atom? (You may need to use the scrollbar to see all the choices.) Explanation Check 10 (choose one) (choose one) linear bent T-shaped trigonal planar trigonal pyramidal square planar square pyramidal tetrahedral sawhorse trigonal bipyramidal octahedral Q Search 0/3 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center www.OTAPETY Accessibiarrow_forward
- Answer the questions in the table below about the shape of the sulfur tetrabromide (SBr_(4)) molecule. How many electron groups are around the central sulfur atom? Note: one "electron group" means one lone pair, one single bond, one double bond, or one triple bond. What phrase best describes the arrangement of these electron groups around the central sulfur atom? (You may need to use the scrollbar to see all the choices.arrow_forwardA 0.02852 g sample of gas occupies 10.0 mL at 291.5 K and 1.10 atm. Upon further analysis, the compound is found to be 38.734% C and 61.266% F. What is the molecular formula and lewis structure of the compound?arrow_forwardOo.10. Subject:- Chemistryarrow_forward
- Predicting deviations from ideal bond angles Consider the carbonate (co-). anion. What is the central atom? Enter its chemical symbol. How many lone pairs are around the central atom? What is the ideal angle between the carbon-oxygen bonds? Compared to the ideal angle, you would expect the actual angle between the carbon-oxygen bonds to be ... 0 口。 (choose one) (choose one) about the same bigger smallerarrow_forwardAnswer the questions in the table below about the shape of the phosphorus pentabromide (PBr.) molecule,. How many electron groups are around the central phosphorus atom? Note: one "electron group" means one lone pair, one single bond, one double bond, or one triple bond. What phrase best describes the arrangement of these electron groups around the central phosphorus atom? (You may need to use the scrollbar to see all the choices.) (choose one) くarrow_forwardPredicting deviations from ideal bond angles Consider the nitrogen trifluoride (NF3) molecule. What is the central atom? Enter its chemical symbol. How many lone pairs are around the central atom? What is the ideal angle between the nitrogen-fluorine bonds? Compared to the ideal angle, you would expect the actual angle between the nitrogen-fluorine bonds to be ... 0 口。 (choose one) (choose one) about the same bigger smallerarrow_forward
- Use VSEPR to predict the geometry (including bond angles) about each interior atom of methyl azide (CH3N3) and draw the molecule. Would you expect the bond angle between the two interior nitrogen atoms to be the same or different? Would you expect the two nitrogen–nitrogen bond lengths to be the same or different?arrow_forwardELECTRONIC STRUCTURE AND CHEMICAL BONDING Predicting deviations from ideal bond angles Consider the chlorite (C102) a anion. What is the central atom? Enter its chemical symbol. How many lone pairs are around the central atom? What is the ideal angle between the chlorine-oxygen bonds? Compared to the ideal angle, you would expect the actual angle between the chlorine-oxygen bonds to be... 0 0 (choose one) X 3 1/5arrow_forward= O ELECTRONIC STRUCTURE AND CHEMICAL BONDING Predicting deviations from ideal bond angles Consider the ammonium (NH) cation. What is the central atom? Enter its chemical symbol. How many lone pairs are around the central atom? 0 What is the ideal angle between the nitrogen- hydrogen bonds? Compared to the ideal angle, you would expect the actual angle between the nitrogen-hydrogen bonds to be... 0. (choose one) X 0/5arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning