
Chemistry for Engineering Students
4th Edition
ISBN: 9781337398909
Author: Lawrence S. Brown, Tom Holme
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:ING AND MOLECULAR STRUCTURE
Previous Page
16 of 16
Reforences
Use the References to access important values if needed for this question.
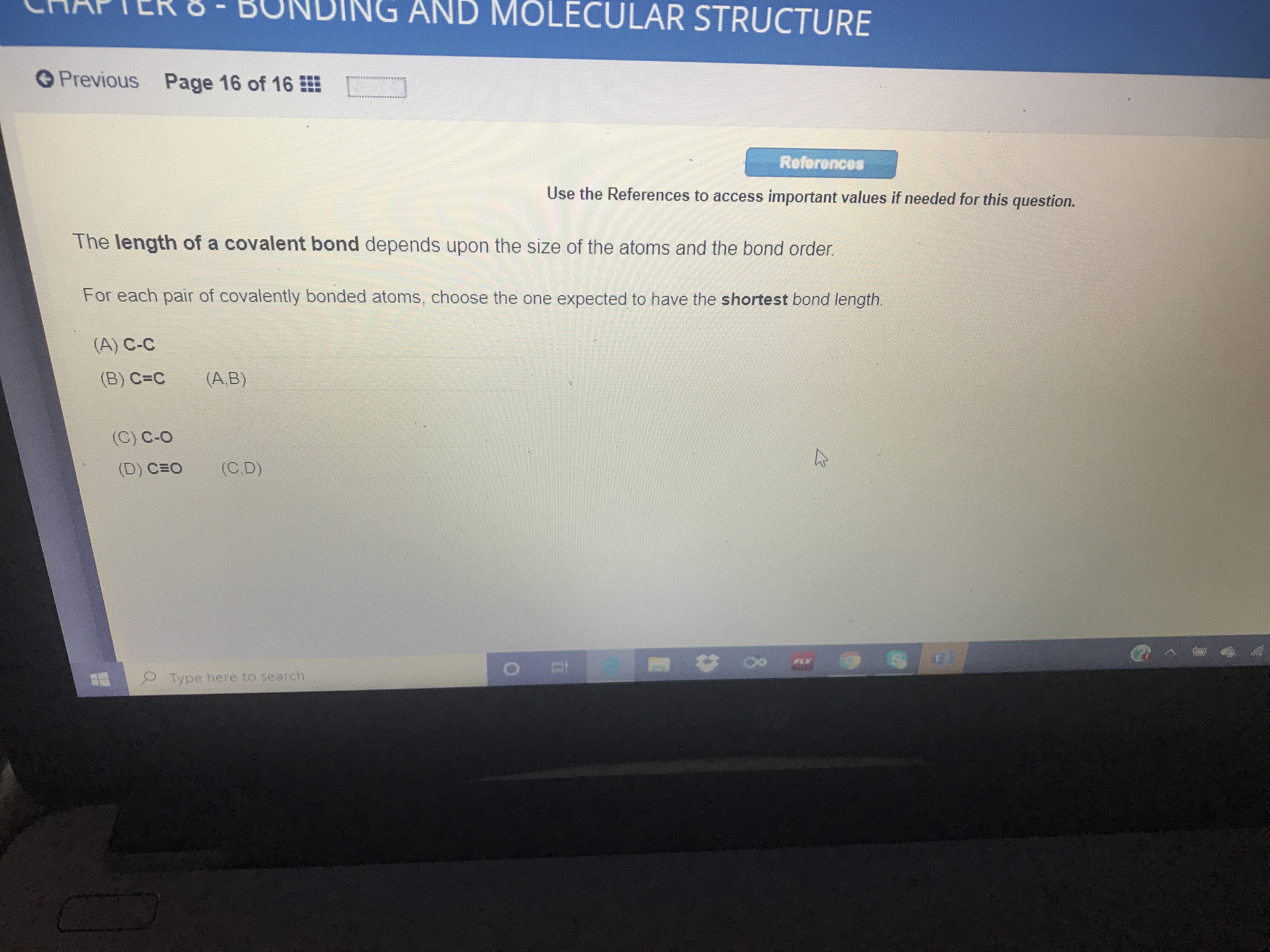
The length of a covalent bond depends upon the size of the atoms and the bond order.
For each pair of covalently bonded atoms, choose the one expected to have the shortest bond length.
(A) C-C
(B) C=C
(A,B)
(C) C-O
(D) CEO
(C.D)
FLV
Type here to search
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Similar questions
- 7.30 The bond in HF is said to be polar, with the hydrogen carrying a partial positive charge. For this to be true, the hydrogen atom must have less than one electron around it. Yet the Lewis dot structure of HF attributes two electrons to hydrogen. Draw a picture of the electron density distribution for HF and use it to describe how the hydrogen atom can carry a partial positive charge. How can these two models of the HF bond (the electron density and the Lewis structure) seem so different and yet describe the same thing?arrow_forward7.53 How do sigma and pi bonds differ? How are they similar?arrow_forwardGiven the bonds C N, C H, C Br, and S O, (a) which atom in each is the more electronegative? (b) which of these bonds is the most polar?arrow_forward
- 7.50 Chemical species are said to be isoelectronic if they have the same Lewis structure (regardless of charge). Consider these ions and write a Lewis structure for a neutral molecule that is isoelectronic with each of them, (a) CN , (b) NH4+ . (c) CO32arrow_forward7.63 What physical concept forms the premise of VSEPR theory?arrow_forward7.40 Why is it impossible for hydrogen to be the central atom in the Lewis structure of a polyatomic molecule?arrow_forward
- Arrange the following bonds in order of increasing polarity using electronegativities of atoms: PO, CCl, AsBr.arrow_forwardCHAPTER 8 - BONDING AND MOLECULAR STRUCTURE Previous Page 15 of 16 Next O References Use the References to access important values if needed for this question. The length of a covalent bond depends upon the size of the atoms and the bond order. For each pair of covalently bonded atoms, choose the one expected to have the shortest bond length. (A) P-Br (B) C-Br (A.B) (C) P-I (D) P-CI (C.D) Type here to search FLVarrow_forwardThe strength of a covalent bond depends upon the size of the atoms and the bond order. In general short bonds are strong bonds. For each pair of covalently bonded atoms, choose the one expected to have the higher bond energy. (A) CEO (B) C=O (C) C-N (D) CEN Use the References to access important values if needed for this question. (A,B) (C,D)arrow_forward
- The strength of a covalent bond depends upon the size of the atoms and the bond order. In general short bonds are strong bonds. For each pair of covalently bonded atoms, choose the one expected to have the higher bond energy. (A) C-C (B) CEC (C) C=O (D) C-O Use the References to access important values if needed for this question. (A,B) (C,D)arrow_forwardThe strength of a covalent bond depends upon the size of the atoms and the bond order. In general short bonds are strong bonds. For each pair of covalently bonded atoms, choose the one expected to have the higher bond energy. (A) C-C (B) CEC (C) N-N (D) NEN (A,B) (C,D)arrow_forwardThe strength of a covalent bond depends upon the size of the atoms and the bond order. In general short bonds are strong bonds. For each pair of covalently bonded atoms, choose the one expected to have the higher bond energy. (A) N=N (B) N-N (C) CEC (D) C=C Use the References to access important values if needed for this question. (A,B) (C,D)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning