
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
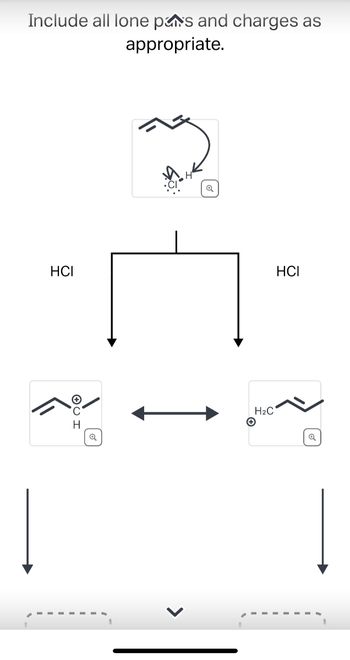
Transcribed Image Text:Include all lone pas and charges as
appropriate.
HCI
✪
H
Q
H₂C
HCI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Remaining Time: 31 minutes, 00 seconds. ¥ Question Completion Status: A Moving to another question will save this response. Question 6 Arrange the following in order of decreasing electronegativity (eg A>B>C}: Mo, Ge, Ba, S, Si, Sr A Moving to another question will save this response. F10 Prisc F2 F3 てい。 Esc F4 F5 Scr Lk & 23 2$ 2 9. R. E gel A Caps Lockarrow_forwardWhich bond has the least polarity? O Si-P O Si-S O S-Se O Si-CI O S-Iarrow_forwardIdentify the cyanide ion. Answer Choices in attached imagearrow_forward
- Which of the following is most likely a polar covalent bond? O Na-Cl O C-N OH-H OK-F O Ca-Oarrow_forwardExercise 9.57 Diaw the dipole arrow that represents the dipole moment of the Lewis structure 向 No clements selected :C=0: Select the elements from the list and add them to the canvas setting the appropriate attributes.arrow_forwardWhat is the correct lewis structure of formaldehyde, H₂CO? 1 H H H H H H :O: C :Ö: C H H H Harrow_forward
- How many polar covalent bonds are present in the following molecule?arrow_forwardA student proposes the following Lewis structure for the water (H,0) molecule. H -0 -H Assign a formal charge to each atom in the student's Lewis structure. atom formal charge left H right H Continue MacBook Pr % %23arrow_forwardDetermine the formula unit for the compound formed when each pair of ions interacts. Al³+ and CN: Ca²+ and SO: Lit and NO3: NH‡ and F¯:arrow_forward
- Cc.67.arrow_forwardents V Soul (TV series) O REPRESENTATIONS OF ORGANIC MOLECULES Drawing a skeletal structure from a Lewis structure Convert the Lewis structure below into a skeletal structure. Н—С— Н H. H- H- Н—С—Н H. HH H. H- Н—С—С—С—С—С—С—С- Н C=C H- H. H. H. Н—С— Н H. ct+ Click and drag to start drawing a structure. HIC CIH HICarrow_forwardWhich is a polar covalent bond? O C-F OH-H CEC C-C O C=C A Moving to another question will save this resparrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY