
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
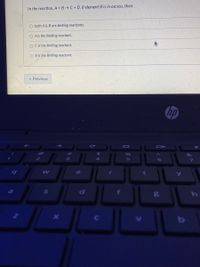
Transcribed Image Text:In the reaction, A + B →C+ D, if element B is in excess, then:
O both A & B are limiting reactants.
O A is the limiting reactant.
O Cis the limiting reactant.
O B is the limiting reactant.
« Previous
hp
%23
24
&
4
5
e
Expert Solution
arrow_forward
Step 1
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Honors Chemistry Chapter 9 Name/Date 3. Gold is very valuable because it does not oxidize easily. It can be oxidized by reacting it with nitric acid as shown below: Au(s) + 4H'(aq) + NO: (aq) → NO(g) + 2H:0(g) + Au³ (aq) 3a. If 30 grams of gold react with 3.4 grams of nitrate ion, which reactant is limiting?. 3b. What is the theoretical yield?. 4. Lead (II) nitrate reacts with sodium chloride according to the equation below: Pb(NO.): + NaCl → PbCla + NANO. 4a. If 8.3 grams of lead (II) nitrate react with 5 grams of sodium chloride, which material would be the limiting reactant? 4b. What is the theoretical yield?arrow_forwardBalance the following chemical equation and determine the number of moles of Fe(s) produced when 1 moles of Fe2O3 (s) react with excess C(s) to produce Fe(s) and CO₂ (g). Fe2O3(s) + C(s) → Fe(s) + CO₂(g)arrow_forwardIn a lab, a grade 11 chemistry student used 0.0123 mol of solid magnesium metal. The student proceeds to burn the magnesium metal in a Bunsen burned with an excess amount of oxygen gas to produce 0.41g of magnesium oxide based on the following balanced equation: 2Mg(s) + 02(g) —> 2MgO(s). The theoretical amount of magnesium oxide should be what?arrow_forward
- There are two steps in the extraction of copper metal from chalcocite, a copper ore. In the first step, copper(I) sulfide and oxygen react to form copper(I) oxide and sulfur dioxide: 2 Cu₂ S(s) + 3 0₂(g) →2 Cu₂O(s) +2 SO₂ (g) In the second step, copper(I)oxide and carbon react to form copper and carbon monoxide: Cu₂O(s) + C(s)→2 Cu(s)+CO(g) Write the net chemical equation for the production of copper from copper(I) sulfide, oxygen and carbon. Be sure your equation is balanced. ローロ 06arrow_forwardChloroform, CHCl3, reacts with chlorine, Cl2, to form carbon tetrachloride, CCl4, and hydrogen chloride, HCl. In an experiment 25 grams of chloroform and 0.36 mol of chlorine were mixed. Which is the limiting reactant? The equation is given as CHCl3 + Cl2 = CCl4 + HClarrow_forward[Tutorial: Limiting reactant stoichiometry] This question will walk you through the steps of calculating the mass of products produced based on your determination of the limiting reactant. b) Step 2a: Use dimensional analysis to determine the theoretical yield of the product. Calculate the theoretical yield in grams Al₂O₃ from the complete reaction of 64.7 grams Al according to the following balanced chemical equation: 2 Al(s) + Fe₂O₃(s) → Al₂O₃(s) + 2 Fe(s) c) Calculate the theoretical yield in grams Al₂O₃ from the complete reaction of 201 grams Fe₂O₃ according to the following balanced chemical equation: 2 Al(s) + Fe₂O₃(s) → Al₂O₃(s) + 2 Fe(s) d) Which of the following substances is the limiting reactant? e) What is the mass in grams of the excess Fe₂O₃ remaining after the partial reaction of 201 g Fe₂O₃ with 64.7 g Al? Give your answer to three significant figures.arrow_forward
- Balance the following chemical equation (using the number "1" if the blank normally is left unfilled). Na (s) + NaF (s) F₂ (g) ---->arrow_forwardWhen exposed to air, aluminum metal, AlAl, reacts with oxygen, O2O2, to produce a protective coating of aluminum oxide, Al2O3Al2O3, which prevents the aluminum from rusting underneath. The balanced reaction is shown here: 4Al+3O2→2Al2O3 a) What is the theoretical yield of aluminum oxide if 3.00 molmol of aluminum metal is exposed to 2.55 molmol of oxygen? Express your answer with the appropriate units.arrow_forwardConsider the following balanced equation. 3 Ag(s) + 4 HNO3(aq) → 3 AgNO3(aq) + NO(g) + 2 H2O(l) Calculate the number of grams of NO produced as a byproduct of the reaction of 118.17 grams of Ag with excess HNO3. Give your answer to the correct number of significant figures without unit. Molar mass of Ag: 107.87 g/mol Molar mass of HNO3: 63.01 g/mol Molar mass of AgNO3: 169.87 g/mol Molar mass of NO: 30.01 g/mol Molar mass of H2O: 18.02 g/molarrow_forward
- According to the following reaction, how many grams of nitrogen monoxide will be formed upon the complete reaction of 24.6 grams of oxygen gas with excess nitrogen gas? N₂ (g) + O₂ (g) →→→2NO (g) grams nitrogen monoxidearrow_forwardThe useful metal manganese can be extracted from the mineral rhodochrosite by a two-step process. In the first step, manganese(II) carbonate and oxygen react to form manganese(IV) oxide and carbon dioxide: 2MnCO3(s) + O₂(g) → 2MnO₂ (s) + 2 CO₂ (g) In the second step, manganese (IV) oxide and aluminum react to form manganese and aluminum oxidide: 3MnO₂ (s) + 4A1(s) 3 Mn(s) + 2 Al₂O3(s) - Suppose the yield of the first step is 73.% and the yield of the second step is 71.%. Calculate the mass of manganese(II) carbonate required to make 8.0 kg of manganese. Be sure your answer has a unit symbol, if needed, and is rounded to 2 significant digits. do 0 x10 Ar ㅁㅁ X D Garrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY