
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
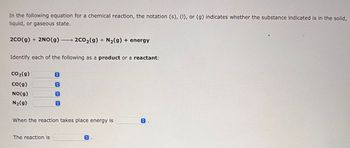
Transcribed Image Text:In the following equation for a chemical reaction, the notation (s), (I), or (g) indicates whether the substance indicated is in the solid,
liquid, or gaseous state.
2CO(g) + 2NO(g) → 2CO₂(g) + N₂(g) + energy
Identify each of the following as a product or a reactant:
CO₂(g)
CO(g)
NO(g)
N₂(g)
©
O
The reaction is
C
When the reaction takes place energy is
O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Volume of cool water (mL) Volume of hot water (mL) Initial temperature of cool water (°C) Initial temperature of hot water (°C) Final temperature of water in calorimeter (°C) Temperature change for cool water (°C) Temperature change for calorimeter (°C) Temperature change for hot water (°C) Heat change for cool water (J) Heat change for hot water Heat change for calorimeter (J) Heat capacity of calorimeter (J/°C) Trial 1 50.0 47.5 24.3 38.9 37.0 Trial 2 50.0 46.1 24.9 35.0 33.7 Trial 3 50.0 44.8 23.3 33.8 30.8arrow_forwardQuestion 4 Which of the following changes is an exothermic chemical reaction? combustion of methane B condensation of steam C decomposition of sodium chloride evaporation of waterarrow_forwardUse the References to access important values if needed for this question Ethanol, C2H0, is most often blended with gasoline - usually as a 10 percent mix - to create a fuel called gasohol. Ethanol is a renewable resource and ethanol-blended fuels, like gasohol, appear to bum more efficiently in internal combustion engines. The combustion enthalpy of ethanol is 1366.9 kJ/mol The combustion enthalpy of 2-methylpentane, C6H14 is 4.157x103 kJ/mol. Calculate the energy released during the complete combustion of 382 g 2-methylpentane kJ Assuming the same efficiency, would 382 g ethanol provide more, less, or the same quantity of energy as 382 g 2-methylpentane? | Submit Answer Retry Entire Group 8 more group attempts remainingarrow_forward
- Hydrogen cyanide (HCN) is prepared from ammonia (NH3) and natural gas (CH4) as shown in the equation below. How many grams of H₂O form when 1,413 kJ of heat is formed? 2NH3(g) + 302(g) + 2CH4 → 2HCN(g) + 6H₂O(g) ΔΗ = -939 kJarrow_forwardIn the following equation for a chemical reaction, the notation (s), (1), or (g) indicates whether the substance indicated is in the solid, liquid, or gaseous state. 2NO(g) + 2H2(g) →N2(g) + 2H,O(M) + energy Identify each of the following as a product or a reactant: H2O)| NO(g) N2(g) H2(g) When the reaction takes place energy is The reaction isarrow_forwardIn the following equation for a chemical reaction, the notation (s), (l), or (g) indicates whether the substance indicated is in the solid, liquid, or gaseous state.2C2H6(g) + 7O2(g) 4CO2(g) + 6H2O(g) + energyIdentify each of the following as a product or a reactant: CO2(g) _________product/reactant C2H6(g) _________product/reactant O2(g) _________product/reactant H2O(g) _________product/reactantarrow_forward
- In the following equation for a chemical reaction, the notation (s), (1), or (g) indicates whether the substance indicated is in the solid, liquid, or gaseous state. CO(g) + H₂O(l) + energy CO₂(g) + H₂(g) Identify each of the following as a product or a reactant: H₂O(1) H₂(g) CO₂(g) CO(g) 0 The reaction is O When the reaction takes place energy is O. Karrow_forwardConsider the process of converting liquid water to steam: H20 (1) → H20 (g) What is true for this reaction? Select all that are true. It absorbs heat It has a -AH ΔΗ-0 It has a +AH It is endothermic It is neither exothermic nor endothermic It releases heat It is exothermicarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY