
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
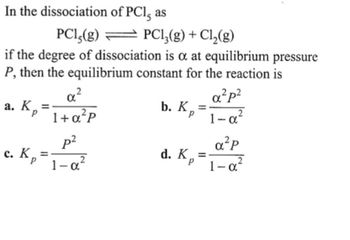
Transcribed Image Text:In the dissociation of PCI, as
PCl5(g)
PC₁₁(g) + C₁₂(g)
if the degree of dissociation is a at equilibrium pressure
P, then the equilibrium constant for the reaction is
a²p²
a. Kp
b. K,
=
1+α²P
1-α-
p²
a²P
c. K =
1-a²
d. Kp =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- At 300 K the reaction 2 BrCl(g)<--> Br(g) + Cl(g) has K = 377. What is the value of K for the reaction Br(g)+Cl(g)<-> 2 BrCl(g)?arrow_forwardConsider the reaction: S(s) + O2(g); SO2(g) Write the equilibrium constant for this reaction in terms of the equilibrium constants, Ką and K,, for reactions a and b below: а.) 2 S(8) + 3 О2(g) 2 SO3(g) Ka b.) SO2(g) + 1/2 02(g) SO3(g) K, K =arrow_forwardConsider the reaction: CH3COOH (aq) + H₂O(1) K = 1.8 x 10-5 at 25 °C Part A If a solution initially contains 0.225 mol L-¹ CH3COOH, what is the equilibrium concentration of H3O+ at 25 °C? Express your answer in moles per litre to two significant figures. 15. ΑΣΦ [H3O+] = H3O+(aq) + CH3COO (aq) Submit Request Answer ? mol L-1arrow_forward
- -2 The equilibrium constant, K, for the following reaction is 1.71x10 at 1120 K 25O₂(g) 250₂(g) + O₂(9) Calculate K, at this temperature for the following reaction SO₂(g) SO₂(g) + 1/2O₂(g)arrow_forward6. Give the equilibrium constant for the following reaction. 2PB1, (g) + 3Cl,(s) 2PCI;(g) + 3Br,(g)arrow_forward1.arrow_forward
- Consider the reaction: C(s) + O2(g)CO2(g) Write the equilibrium constant for this reaction in terms of the equilibrium constants, K, and K,. for reactions a and b below: a.) C(s) + 1/2 O2(g) Co(g) Ka b.) CO(g) + 1/2 O2(g): Co2(g) K, K=arrow_forwardAt 503 K the equilibrium constant K. for the dissociation of N,O4, N204(g) 2 2 NO2(g) has the value K = = 40.0. Calculate the concentration of N204 left undissociated when 1.00 mol of this gas is heated to 503 K in a 10.0 L container.arrow_forwardAt 1000.0 K, the equilibrium constant for the Reaction (1) is is K1 = 0.016. (1) 2 NO (g) + Br2 (g) = 2 NOB1 (g) Calculate K for the Reaction (2): (2) NOB1 (g) = NO (g) + 1/2 Br2 (g). 7.9 a. O b. 125 O. 62.5 Od. 1.6 x 10-4 0.008 е.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY