
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
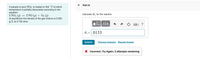
Transcribed Image Text:Part A
A sample of pure NO2 is heated to 334 °C at which
temperature it partially dissociates according to the
equation
2 NO2 (g) = 2 NO (g) + O2 (g)
At equilibrium the density of the gas mixture is 0.520
g/L at 0.750 atm .
Calculate K. for the reaction.
Π ΑΣφ.
K = .0133
Submit
Previous Answers Request Answer
Incorrect; Try Again; 3 attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Ammonia has been studied as an alternative "clean" fuel for internal combustion engines, since its reaction with oxygen produces only nitrogen and water vapor, and in the liquid form it is easily transported. An industrial chemist studying this reaction fills a 1.5 L flask with 1.6 atm of ammonia gas and 2.6 atm of oxygen gas, and when the mixture has come to equilibrium measures the partial pressure of nitrogen gas to be 0.48 atm. Calculate the pressure equilibrium constant for the combustion of ammonia at the final temperature of the mixture. Round your answer to 2 significant digits. K -0arrow_forwardThe equilibrium constant, Kc, for the reactionPCl3 (g) + Cl2 (g) <---->PCl5 (g)equals 14 at 800 °C. If 0.500 mol each of phosphorus trichloride and chlorine are added to a 5.0 L reaction vessel, what is the equilibrium composition of the mixture at 230 °C?arrow_forwardPhosphorous pentachloride decomposes according to the reaction PCI, (g) PCI, (g) + Cl₂(g) A 12.3 g sample of PCI, is added to a sealed 1.50 L flask and the reaction is allowed to come to equilibrium at a constant temperature. At equilibrium, 33.6% of the PCI, remains. What is the equilibrium constant, K., for the reaction? Ke 0.0604 Incorrectarrow_forward
- Phosphorus pentachloride decomposes according to the chemical equation PCI, (g) PC13(g) + Cl₂(g) K = 1.80 at 250 °C A 0.4044 mol sample of PCI, (g) is injected into an empty 4.40 L reaction vessel held at 250 °C. Calculate the concentrations of PCI, (g) and PCI, (g) at equilibrium.arrow_forwardConsider the following equilibrium: 2NOCl (g) <--> 2NO (g) + Cl2 (g) With K = 1.6 x 10-5. 1.00 mole of pure NOCl and 0.964 mole of pure Cl2 are placed in a 1.00 L container. Calculate the equilibrium concentration of Cl2 (g).arrow_forwardSteam reforming of methane ( CH, ) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. An industrial chemist studying this reaction fills a 5.0 L flask with 0.93 atm of methane gas and 2.7 atm of water vapor, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 0.47 atm. Calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. Round your answer to 2 significant digits. K_ = || x10arrow_forward
- Suppose a 250. mL flask is filled with 1.2 mol of Br,, 1.4 mol of OCl, and 1.1 mol of BrCl. The following reaction becomes possible: Br, (g) +OCl, (g) - BrOC1 (g) +BrC1(g) The equilibrium constant K for this reaction is 0.483 at the temperature of the flask. Calculate the equilibrium molarity of OCl,. Round your answer to two decimal places. OMarrow_forwardThe equilibrium constant K. for the reaction H2(g) + Br2(g) == 2HB1(g) is 2.18 × 10° at 730°C. Starting with 3.20 moles of HBr in a 12.0-L reaction vessel, calculate the concentrations of H2, Br2, and HBr at equilibrium.arrow_forwardThe equilibrium constant, K, for the following reaction is 6.59×10-2 at 546 K. PCl5 (g) PCl3 (g) + Cl2 (g) An equilibrium mixture of the three gases in a 6.59 L container at 546 K contains 0.254 M PCl5, 0.129 M PCl3 and 0.129 M Cl2. What will be the concentrations of the three gases once equilibrium has been reestablished, if the volume of the container is increased to 11.7 L?arrow_forward
- Give detailed solutionarrow_forwardPhosphorus pentachloride decomposes according to the chemical equation PCI, (g) PCI, (g) + CL, (g) K. = 1.80 at 250 °C %3! A 0.3251 mol sample of PCI, (g) is injected into an empty 3.60 L reaction vessel held at 250 "C. Calculate the concentrations of PCI, (g) and PCI, (g) at equilibrium.arrow_forward6. Hydrogen gas is finding more acceptance as an alternative fuel. It is produced along with carbon dioxide by the reaction of carbon monoxide and gaseous water at 915 K. At this temperature, the equilibrium constant (K.) is 1.56. If this reaction is started in a 4.00 L closed system flask with 0.450 mol of CO, 1.25 mol H2O, 1.15 mol CO2, and 3.50 mol H2, calculate the concentrations of all substances in the flask initially and at equilibrium. Put these values in the ICE table below. (Hint: you will need to use the quadratic formula.) CO Н20 CO2 H2 INITIAL CHANGE EQUILIBRIUM 0.1125 0.3125 0.2875 0.875 -X -X +X +x e these values in the table. d. Calculate the reaction quotient, Q. e. Express the direction of equilibrium shift as an algebraic expression. Complete the change row of the table. f. Substitute the algebraic expressions into the equilibrium expression and solve for the equilibrium concentrations. Place these values in the table.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY