
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Please help,
Will provide helpful ratings for correct solution. Thank u
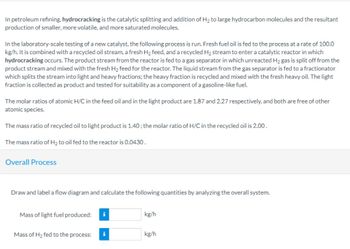
Transcribed Image Text:In petroleum refining, hydrocracking is the catalytic splitting and addition of H₂ to large hydrocarbon molecules and the resultant
production of smaller, more volatile, and more saturated molecules.
In the laboratory-scale testing of a new catalyst, the following process is run. Fresh fuel oil is fed to the process at a rate of 100.0
kg/h. It is combined with a recycled oil stream, a fresh H₂ feed, and a recycled H₂ stream to enter a catalytic reactor in which
hydrocracking occurs. The product stream from the reactor is fed to a gas separator in which unreacted H₂ gas is split off from the
product stream and mixed with the fresh H₂ feed for the reactor. The liquid stream from the gas separator is fed to a fractionator
which splits the stream into light and heavy fractions; the heavy fraction is recycled and mixed with the fresh heavy oil. The light
fraction is collected as product and tested for suitability as a component of a gasoline-like fuel.
The molar ratios of atomic H/C in the feed oil and in the light product are 1.87 and 2.27 respectively, and both are free of other
atomic species.
The mass ratio of recycled oil to light product is 1.40; the molar ratio of H/C in the recycled oil is 2.00.
The mass ratio of H₂ to oil fed to the reactor is 0.0430.
Overall Process
Draw and label a flow diagram and calculate the following quantities by analyzing the overall system.
Mass of light fuel produced: i
Mass of H₂ fed to the process:
i
kg/h
kg/h
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Similar questions
- How about the other parts? Can you summarize the final answers by listing them down at beginning?arrow_forwardDiscuss challenges and obstacles to implementation of microservices architecture in the enterprise.arrow_forwardAnswer was extremely helpful, can you answer the rest of the parts?arrow_forward
- What is the best method for estimating the distribution of nonkey components at the actual (operating) reflux?arrow_forwardWhat is the limitation of a single-section cascade?arrow_forward1. Briefly compare the three levels of diagrams for chemical processes please include their main use and key components.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The