
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
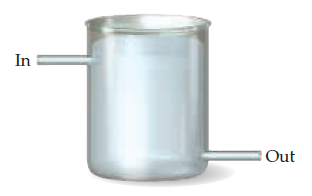
In a
of a solution in an apparatus as illustrated. A solution is
continuously flowing into the apparatus at the top and out at
the bottom, such that the amount of solution in the apparatus
is constant with time. (a) Is the solution in the apparatus
a closed system, open system, or isolated system? (b) If the inlet
and outlet were closed, what type of system would it be?
Transcribed Image Text:In
Out
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The freezing point of ethanol, CH,CH,OH, is -117.30°C at 1 atmosphere. A nonvolatile, nonelectrolyte that dissolves in ethanol is saccharin . A student dissolves 11.77 grams of saccharin, C,H&NO;S (183.2 g/mol), in 287.1 grams of ethanol. Use the table of boiling and freezing point constants to answer the questions below. Solvent Formula Kp (°C/m) Kf (°C/m) Water H2O 0.512 1.86 Ethanol CH;CH2OH 1.22 1.99 Chloroform CHCI3 3.67 Benzene C,H6 2.53 5.12 Diethyl ether CH;CH2OCH2CH3 2.02 The molality of the solution is m. The freezing point of the solution is °C.arrow_forwardWhat is the osmotic pressure (in atm) of a 1.56 M aqueous solution of urea Round your answer to 3 significant digits. II = atm x10 X Ś (NH,),CO] at 16.0 °C?arrow_forwardA pharmacist mixes togehter 20 g of crystals of compound A and 10g of crystals of compound B. The mixture is then dissolved in 120mL of water to make cough syrup.arrow_forward
- Phenanthrene, C14H10, is an aromatic hydrocarbon. Some phenanthrene is dissolved in 50.0 g of benzene and the boiling point of the resulting solution is 80.51°C. What mass of phenanthrene must have been dissolved? The Kb for benzene is 2.53 °C/m and the boiling point of pure benzene is 80.2°C.arrow_forwardWhich of the following would be expected to have the highest solubility in water? Η Η H¬C-C-O-H Η Η O AgCl (ethanol) H₂S AgCl Η Η ΗΙ Η Η | | H=C=C=C-C-C-H | | | Η Η Η Η (pentane) Ηarrow_forward5.152g of Na2CO3(s) is added to 75.0mL of water at 21.20°C. When the dissolution is complete, the final temperature of the solution is measured at 25.00°C. The final volume of the solution is 75.8mL, and the density of water at 21.20°C is 0.99798g/mL. Identify the strongest intermolecular force present in Na2CO3(s): and in water:arrow_forward
- The boiling point of chloroform, CHCl3, is 61.70°C at 1 atmosphere. A nonvolatile, nonelectrolyte that dissolves in chloroform is aspirin.A student dissolves 14.17 grams of aspirin, C9H8O4 (180.1 g/mol), in 238.1 grams of chloroform. Use the table of boiling and freezing point constants to answer the questions below. Solvent Formula Kb (°C/m) Kf (°C/m) Water H2O 0.512 1.86 Ethanol CH3CH2OH 1.22 1.99 Chloroform CHCl3 3.67 Benzene C6H6 2.53 5.12 Diethyl ether CH3CH2OCH2CH3 2.02 The molality of the solution is ____________ m.The boiling point of the solution is _________________°C.arrow_forwardIf dissolving 1.5 g of a solute into 100 mL of water caused the temperature of the solution toincrease by 4.7 ⁰C, what would the change of temperature be if 3.0 g of the solute were dissolvedin the same volume of water? Explain.arrow_forwardIt is desired to prepare 2.5 m^3 of a 60 mol percent methanol - water solution. Determine the volumes of methanol and water required to be mixed at ambient temperature given that the partial molar volumes of methanol and water are 58.3x10^-6m^3/mol and 17.2 x 10 m^3/mol respectively. The density of the methanol is 782.51 kg/m^3. Do not use chatgpt. Thank you!arrow_forward
- If dissolving 1.5 g of a solute into 100 mL of water caused the temperature of the solution toincrease by 4.7 ⁰C, what would the change of temperature be if 3.0 g of the solute were dissolvedin the same volume of water? Explain.arrow_forward0.0471 kg of biphenyl (C12H10) is dissolve in benzene (C&Hs) to create a solution with a total volume of 350.0 mL. (Assume the change in volume is negligible) If the boiling point of pure benzene is 80.1 °C, then what would be the boiling point of this solution in °C? (Kb for benzene is 2.53 °C/m and the density of benzene is 0.877 g/mL)arrow_forwardThe boiling point of diethyl ether, CH3 CH₂ OCH2 CH3, is 34.50 °C at 1 atmosphere. A nonvolatile, nonelectrolyte that dissolves in diethyl ether is chlorophyll. A student dissolves 11.24 grams of chlorophyll, C55 H72 MgN4O5 (893.5 g/mol), in 249.4 grams of diethyl ether. Use the table of boiling and freezing point constants to answer the questions below. Solvent Water Ethanol Chloroform Benzene Formula H₂O CH3 CH₂OH CHC13 C6H6 Diethyl ether CH3 CH₂ OCH₂ CH3 The molality of the solution is The boiling point of the solution is Kb (°C/m) Kf (°C/m) 1.86 1.99 0.512 1.22 3.67 2.53 2.02 m. °C. 5.12arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY