
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
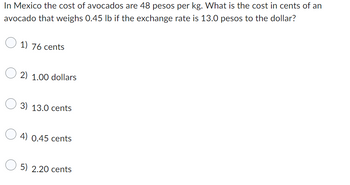
Transcribed Image Text:In Mexico the cost of avocados are 48 pesos per kg. What is the cost in cents of an
avocado that weighs 0.45 lb if the exchange rate is 13.0 pesos to the dollar?
1) 76 cents
2) 1.00 dollars
3) 13.0 cents
0.45 cents
5) 2.20 cents

Transcribed Image Text:Daniel's recipe for onion soup calls for 4.0 lb of thinly sliced onions. If one onion has
an average mass of 115 g, how many onions does Daniel need?
1) 16
2) 2
3) 29
4) 10
5) 5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hand written solutions are strictly prohibitedarrow_forwardThe concentration of carbon monoxide (CO), a common air pollutant, is found in a room to be 5.7 x 10-3 mg/cm3. How many grams of CO are present in the room if the room's dimensions measure 3.5m x 3.0m x 3.2m?arrow_forwardGaseous ethane (CH,CH,) will react with gaseous oxygen (O2) to produce gaseous carbon dioxide (CO2) and gaseous water (H,O). Suppose 0.601 g of ethane is mixed with 2.9 g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits. g x10 ?arrow_forward
- In the opening scene of the movie Raiders of the Lost Ark, Indiana Jones tries to remove a gold idol from a booby-trapped pedestal. He replaces a gold (D = 19.32 g/cm²³) idol of dimensions 5.0 inches by 5.0 inches by 5.0 inches with a bag of sand of approximately double the volume with sand (D = 3.65 g/cm³). Is the bag of sand really equal to the gold idol or will Dr. Jones set off the trap? Explain your answer and include a mathematical explanation. ML Cm3arrow_forwardThe shattered glass case at the scene of a jewelry store robbery was determined to be made of potash borosilicate glass, which has a density of 2.162g/mL. A 4.034g glass fragment was recovered from a suspect's clothing. When the fragment was placed into a graduated cylinder filled with water, 1.63mL of the water was displaced. Calculate the density of the glass fragment.arrow_forward2. You purchase a rectangular piece of metal that has dimensions 5.0 X 15.0 X 30.0 mm and mass 43.43 g. The seller tells you that the metal is gold. To check this, you compute the average density of the piece. What value do you get? Were you cheated?arrow_forward
- Chlorine gas (C1₂) reacts with solid calcium (Ca) to produce solid calcium chloride (CaCl₂). Write a balanced chemical equation for this reaction. 0 0-0 X 2 5 Sarrow_forwardChalcopyrite is among the most common varieties of copper ore. Assuming chalcopyrite is 34.4% copper by mass (34.4 grams copper per 100. grams of ore), how many kilograms of chalcopyrite would be needed to smelt 30.0 kilograms of pure copper?arrow_forwardSuppose you are measuring the mass of a solid sample on a balance using a weigh boat. You record the data in a table. Mass of weigh boat 3.451 g Mass of weigh boat and sample 7.268 g What is the mass of the solid sample (in g)?arrow_forward
- A student weighed their penny (mass = 2.499 g) and followed the same experimental procedure you did in the lab. They found that the penny contained 0.0980 g of copper, or, 3.92%. Estimate the thickness of the copper coating with the following formula. From the US Mint website: the diameter of a penny is 19.05 mm and the thickness is 1.52 mm. The density of copper is 8.96 g/cm3. TIP: your final answer will have the units of cm (think about what to do first.). mass of Cu volume density of Cu Thickness of Cu Coating = area (diameter 2 T T (diameter) (thickness) O 7.39 x 104 cm O 1.65 x 103 cm O 4.22 x 102 cm O 2.80 x 103 cm O 1.89 x 105 cm O impossible to determinearrow_forwardA chardonnay wine is 13.5 percent by mass alcohol. If we consume 24 fluid oz of wine, and the density of the wine is 0.982 mg/mL, how much alcohol was consumed? 09.6 x 103 g alcohol O 301 g alcohol O 94 g alcohol O 106 g alcohol O 13.3 g alcoholarrow_forwardA sunscreen preparation contains 2.50% by mass benzyl salicylate. If a tube contains 4.0 ounces of sunscreen, determine how many kilograms of benzyl salicylate are needed to manufacture 325 tubes of sunscreen. Conversion: 2.205 lb= 1 kg. 16.0 oz = 1 lbarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY