
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
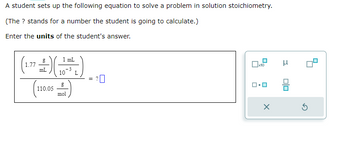
Transcribed Image Text:A student sets up the following equation to solve a problem in solution stoichiometry.
(The ? stands for a number the student is going to calculate.)
Enter the units of the student's answer.
1 mL
(+)(4+2)
mL
3
10 L
1.77
(110.05)
=
20
x10
X
3
00
5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A chemist prepares a solution of iron II chloride FeCl2 by measuring out 3.0g of FeCl2 into a 300.mL volumetric flask and filling to the mark with distilled water. Calculate the molarity of Cl− anions in the chemist's solution. Be sure your answer is rounded to the correct number of significant digits.arrow_forwardA chemist prepares a solution of silver(I) nitrate AgNO3 by measuring out ×6.4102μmol of silver(I) nitrate into a 450. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's silver(I) nitrate solution. Round your answer to 2 significant digits.arrow_forwardA standard acid was prepared by placing 5.98 grams of H2C2O42H2O (molar mass: 126.07 g/mole) into a 250.00-milliliter volumetric flask. H2C2O4 (molar mass: 90.04 g/mole) For the blanks in this question: Report here only your numerical answers (without units), if the value is less than zero as a decimal (not in scientific notation), to the correct number of significant figures. Calculate the moles of H2C2O4 in the flask. Calculate the grams H2C2O4 in the flask. Calculate the molarity of the solution if the volumetric flask is filled to the mark with water.arrow_forward
- A chemist prepares a solution of silver perchlorate (AgClO,) by weighing out 2.40 kg of silver perchlorate into a 500. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in g/dL of the chemist's silver perchlorate solution. Be sure your answer has the correct number of significant digits. x10 đLarrow_forwardYou are making a rubbing alcohol (=isopropyl alcohol) solution to disinfect surfaces. You start with 323.1 mL of a 7.465 M isopropyl alcohol solution, and dilute it with pure water. After the dilution, you end up with a 1.856 M solution. What is the final volume of the solution after the water has been added? Enter your answer in the units of L (liters), do not enter the unit.arrow_forwardA chemist adds 280.0 mL of a 1.32M iron(III) bromide (FeBr,) solution to a reaction flask. Calculate the millimoles of iron(III) bromide the chemist has added to the flask. Round your answer to 3 significant digits. mmol x10arrow_forward
- A chemist makes 250. mL of calcium bromide (CaBr₂) working solution by adding distilled water to 40.0 mL of a 0.767 in water. Calculate the concentration of the chemist's working solution. Round your answer to 3 significant digits. 1 mol x10 X S mol L stock solution of calcium bromidearrow_forwardSuppose 4.20 g of iron(II) chloride is dissolved in 300. mL of a 75.0 m M aqueous solution of silver nitrate. Calculate the final molarity of chloride anion in the solution. You can assume the volume of the solution doesn't change when the iron (II) chloride is dissolved in it. Be sure your answer has the correct number of significant digits. M x10arrow_forwardWhich piece of equipment would you use to measure 7.10 mL of CuSO4 (aq)?arrow_forward
- A chemist adds 435.0 mL of a 0.0630 mmol/L potassium permanganate (KMnO4) solution to a reaction flask. Calculate the mass in milligrams of potassium permanganate the chemist has added to the flask. Round your answer to 3 significant digits.arrow_forwardOne half tsp of table salt (NaCl) weighs aproximately 2.0 grams. (You will need to look up the molar mass for NaCl) There are approximately 237 ml in a cup. Determine the Molarity of the solution 1 cup water with one half tsp table salt. Determine the molarity of this solution using the formula M=g/MM/L. What is the molarity? Show your calculations. (Remember to convert 237 mL to L)arrow_forwardA solution of phosphoric acid is sometimes used to acidify foods, such as jams, to give them a tangy or sour taste. Calculate the amount of phosphoric acid, in moles, that is in 32.8 mL of a 4.46 mol/L phosphoric acid solution. Record your numerical answer with the correct number of significant digits in the first answer box. You do not need to include units as the units appear for you beside the answer box already.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY