
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
In less than 100 words total
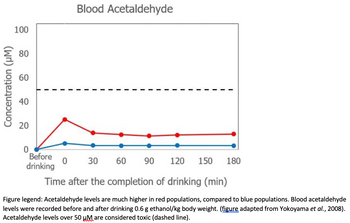
i) describe the physiological reaction of the red population to alcohol consumption, which is usually not seen with the blue population, and
ii) explain in the language of this unit how the enzymatic properties of
Transcribed Image Text:Concentration (HM)
100
80
60
40
20
Before
drinking
Blood Acetaldehyde
0 30
60 90
Time after the completion of drinking (min)
Figure legend: Acetaldehyde levels are much higher in red populations, compared to blue populations. Blood acetaldehyde
levels were recorded before and after drinking 0.6 g ethanol/kg body weight. (figure adapted from Yokoyama et al., 2008).
Acetaldehyde levels over 50 μM are considered toxic (dashed line).
120 150 180
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Similar questions
- (b) The results in the table below were extracted from clinical reports of pediatric age infants (3 – 5 yr.) who had marked lactic acidosis, elevated blood ammonia, poor motor coordination, and showed mental retardation. From skin fibroblast cell cultures, enzyme assays were carried out, providing the results given below. PDH = pyruvate dehydrogenase; E1, E2, E3 signify the three enzyme activities of the PDH complex measured separately. It should be noted that measurement of the overall activity of the PDH complex employs a different assay system than those used for measurement of the activi- ties of the separate, individual component enzymes. Patient or PDH Complex activity nmol/min/mg protein Native E1 E2 Ез Control +dichloroacetate nmol/h/mg protein Patient 1 0.318 + 0.054 0.322 + 0.053 1.54 0.29 3.69 + 1.21 15.1 + 3.0 Patient 2 0.050 ± 0,031 0.044 + 0.027 0.21 + 0.06 6.5 ± 3.1 9.8 ± 1.3 Patient 3 0.092 + 0.022 0.068 + 0.037 0.21 + 0.06 8.48 13.5 + 2.0 Control 1 0.932 + 0.076 1.368…arrow_forwarda) Write the catalytic mechanistic steps used in the urea cycle. If transamination is required, showonly the net reaction of this step.b) Where do the 2 nitrogen atoms and the 1 carbon atom that make the final product come from?arrow_forwardThe protein catalase is an enzyme that catalyzes the decomposition of hydrogen peroxide:2 H2O2 (aq) → 2 H2O (l) + O2 (g)and has a Michaelis-Menten constant of 25 × 10-3 mol·dm-3 and a turnover number of 4.0×107s-1.The total enzyme concentration is 0.016×10-6 mol·dm-3 and the initial substrate concentration is4.32×10-6 mol·dm-3 Calculate the maximum reaction rate (????) for this enzyme, and the initial rateof this reaction. Note that catalase has a single active site.arrow_forward
- Chymotrypsin has the highest affinity for which of the following substrates: Table. The values of KM and kcat for some Enzymes and Substrates Enzyme Chymotrypsin Ки (М) 4.4 x 10-1 8.8 x 10-2 6.6 x 104 Kcat (S-1) 5.1 x 10-2 1.7 x 10-1 1.9 x 102 Substrate N-acetylglycine ethyl ester N-acetylvaline ethyl ester N-acetyltyrosine ethyl ester Catalase H2O2 2.5 x 10-2 1.0 x 107 Urease Urea 2.5 x 10-2 4.0 x 105 OA. N-acetylglycine ethyl ester OB. N-acetylvaline ethyl ester OC. N-acetyltyrosine ethyl ester D. Ureaarrow_forwardi) Re-arrange the Michaelis Menten equation so it involves the ratio [S]. Show all steps beginning Km noting any assumptions or required conditions. Km ii) Calculate the ratio [lo for the case when the rate of product formation is 68% of Vmax and the substrate is in great excess. d[P] dt : k₂ with = [E],[S] Km+[S]' [S]o Km iii) Explain, in a few sentences, why the ratio determines the ratio V Vmax V Vmax Begin by explaining the meaning of stating simply "it's the ratio...." is not sufficient. Include in your explanation the factors that effect v and Vmax. Consider what factors make v different from or equal to Vmax. Consider what Km represents concerning processes involving ES. " iv) Calculate KM at 310K at given the following rate constant information: k₁ = 17 s-¹M-1 at 300K with A = 7300 s-¹M-1 K-1₁ 6 s¹ at 300K with A = 14500 s -1 k₂ = 31 s¹ at 300K with A = 600 s-¹arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON