Question
Can you please help me with this question? I really appreciated
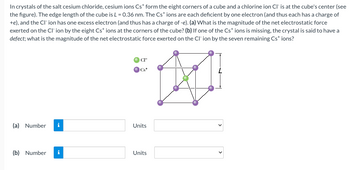
Transcribed Image Text:In crystals of the salt cesium chloride, cesium ions Cs* form the eight corners of a cube and a chlorine ion Cl- is at the cube's center (see
the figure). The edge length of the cube is L = 0.36 nm. The Cs+ ions are each deficient by one electron (and thus each has a charge of
+e), and the CI-ion has one excess electron (and thus has a charge of -e). (a) What is the magnitude of the net electrostatic force
exerted on the CI-ion by the eight Cs* ions at the corners of the cube? (b) If one of the Cs* ions is missing, the crystal is said to have a
defect; what is the magnitude of the net electrostatic force exerted on the CI-ion by the seven remaining Cs* ions?
(a) Number
i
(b) Number i
Cs+
¨Ø
CI
Units
Units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 5 images

Knowledge Booster
Similar questions
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios