
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
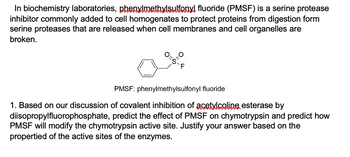
Transcribed Image Text:In biochemistry laboratories, phenylmethylsulfonyl fluoride (PMSF) is a serine protease
inhibitor commonly added to cell homogenates to protect proteins from digestion form
serine proteases that are released when cell membranes and cell organelles are
broken.
=S-F
PMSF: phenylmethylsulfonyl fluoride
1. Based on our discussion of covalent inhibition of acetylcoline, esterase by
diisopropylfluorophosphate, predict the effect of PMSF on chymotrypsin and predict how
PMSF will modify the chymotrypsin active site. Justify your answer based on the
propertied of the active sites of the enzymes.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- What step ends the first-half of the chymotrypsin mechanism? Attack of water on the acyl-enzyme intermediate. Selected Collapse of the first tetrahedral intermediate, releasing of the C-terminal peptide fragment and formation of the acyl-enzyme intermediate. Deprotonation of serine by the histidine. Collapse of the second tetrahedral intermediate leading to the final products. REVIEW QUESTION 1/1 CONTINUE MacBook Pro https://virginiacommonwealth.instructure.com/courses/42082/assignments/271397 esc OTarrow_forwardthe structures of chymotrypsin and other serine proteases revealed that the active sites of these enzymes shared a particular sterochemical arrangement of residues crucial to their activity. This came to be known as the catalytic triad, as it consisted of the oponyomous serine (Ser) residue, along with a histidine (His) and an aspartate (Asp) residue. Which of the following would result in the greatest decrease in function of the catalytic triad found in serine proteases? O Mutation to Ser, Cys, Asp O Mutation to Ser, His, Asp O Mutation to Cys, His, Asp O Mutation to Ser, His, Gluarrow_forwardAzathioprine is an immunosuppressant drug used in kidney transplants. In vivo, azathioprine is metabolized to the hypoxanthine analog 6- mercaptopurine, which is then converted into 6-mercaptopurine ribose monophosphate, the active form of the drug. 6- Mercaptopurine ribose monophosphate also inhibits de novo purine synthesis, reducing uric acid levels in the blood and urine. However, when administered to Lesch – Nyhan patients, there was no effect on uric acid levels. Explain why this is the case.arrow_forward
- Which of the following statements are descriptions of metal ion catalysis or examples of metal ion catalysis? Choose all correct answers a Zn²+ cofactor may properly orient the substrate in the active site through ionic interactions. a covalent bond forms between enzyme and substrate lowers the energy or stabilizes the transition state or intermediate catalyst retains its original form after reaction occurs catalysts may participate in oxidation-reduction reactions by changes in the oxidation statearrow_forwardConsider the metabolic pathway show below that converts substrate A to B with the enzyme A-ase, B to C with B-ase(I), and so forth. Acety/A-ase Acetate - A-ase Deacetylase A-ase Protease & C-ase B-ase ABCD What is the mechanism of regulation of B-ase? Positive allostery Reversible Covalent modification Isoenzymes Proteolytic Activation Feedback Inhibition 1arrow_forwardATP synthase is a protein that catalyzes the formation of the energy storage molecule adenosine triphosphate using adenosine diphosphate and inorganic phosphate. Which of the following are true for the protein, ATP synthase? Select all apply A mutation of Asp61 to glutamic acid would have less of a deleterious effect on its ability to synthesize ATP than a mutation of Asp61 to alanine A mutation of Asp61 to alanine would have less of a deleterious effect on its ability to synthesize ATP than a mutation of Asp61 to glutamic acid The F1 subunit is primarily responsible for translocating H+ ions across the inner mitochondrial membrane during ATP synthesis The FO subunit is primarily responsible for translocating H+ ions across the inner mitochondrial membrane during ATP synthesisarrow_forward
- The purified OXA-M290 enzyme can now be tested to determine which β-lactamase inhibitor is most effective. This inhibitor could be prescribed in combination with a β-lactam antibiotic to treat the infection caused by the E. coli KGH1 strain. Before testing inhibitors against OXA-M290, the kinetic activity of this enzyme must first be measured. The activity of OXA-M290 is measured using nitrocefin, a chromogenic β-lactam antibiotic. When nitrocefin is hydrolyzed by a β-lactamase, it changes from yellow to red in colour. The nitrocefin hydrolysis product has an extinction coefficient of 20,500 M-1 cm-1 at 486 nm. The hydrolysis of 60 μM nitrocefin by 1 nM OXA-M290 is monitored using a microplate reader. The absorbance of the wells in the plate is measured at 486 nm every 30 seconds. This experiment is carried out with three replicates, generating the following data: Time (min) Absorbance of Replicate 1 Absorbance of Replicate 2 Absorbance of Replicate 3 0.5 0.0984…arrow_forwardCompare and contrast the biological roles of the following amino acids the following pairs ofamino acids. Once you have documented these role state which member of the pair is most important and why.arrow_forwardThis image is showing the conformations of ubiquitin superimposed. What do the structures in this figure tell us about the dynamics of enzymes? Discuss the limitations of crystal structures in the analysis of proteins. What are the benefits? What are the advantages of NMR structures?arrow_forward
- A child with coarse facies, corneal clouding, joint stiffness and mental retardation is found to have a Damage to this enzyme directly affects which of partial defect in N-acetylglucosaminotransferase. the following biochemical functions? A. Formation of polysomes B. Microtubule attachment to centrioles C. Synthesis of ribosomal RNA D. Targeting of enzymes for lysosomes E. Transport of sugars into mitochondriaarrow_forwardSmall molecules are used as inhibitors of protein action - as drugs. They most often do this by blocking the active site within the protein. Potential drugs can be screened computationally to determine if they are strongly bound to the protein. Figure 1 shows a possible conformation of a candidate drug molecule, 4-bromo-2- carboxymethylamide-pyrrole (abbreviation: BCMAP) at the active site of a protein (abbreviation: PR). Figure 2 shows the full protein structure whilst figure 3 shows a known inhibitor of the protein at the site, overlayed with another calculated conformer of BCMAP. (a) Explain what types of interactions, both intermolecular and intramolecular, that a molecular mechanics forcefield must be able to describe in order to be able to accurately determine the geometry of BCMAP in the protein. Identify which interactions will be the most important to describe accurately. Figure 1.4-bromo-2-carboxymethylamide-pyrrole (BCMAP) (C, N, O, and Br atoms in yellow, blue, red, and…arrow_forwardThe majority of bacterial mutations that need isoleucine also require valine for growth. Why? Which enzyme or process would be deficient in a mutant that requires solely isoleucine for growth (rather than valine)?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON