
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
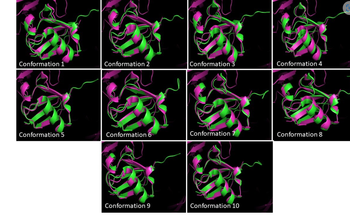
This image is showing the conformations of ubiquitin superimposed. What do the structures in this figure tell us about the dynamics of enzymes? Discuss the limitations of crystal structures in the analysis of proteins. What are the benefits? What are the advantages of NMR structures?
Transcribed Image Text:Conformation 1
Conformation 5
Conformation 2
Conformation 6
Conformation 9
Conformation 3
Conformation 7
Conformation 10
Conformation 4
Conformation 8
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Prepare a schematic diagram and present it as though it were a Figure in a publication (scientificjournal or textbook) (20%). The Figure should illustrate the interactions made between thekey components of (a) total and (b) non-specific binding reactions. In preparing your figure,you should reflect on the role of each of the components of the reaction mixtures, and whythe subtraction of non-specific from total binding allows us to calculate specific binding.arrow_forward5arrow_forwardCan the same pepptide be used in multiple different proteins? yes or no, explain.arrow_forward
- A) Identify the structures that would be found in proteins B) identify the structures that polymerize via condensation reactionsarrow_forwardDuring successful purification of every enzyme, the following may be expected: Select ALL that apply. 1. Solubility in NaCl increases 2. The activity increases 3. The specificity increases 4. The number of subunits increases 5. the epitope number increasesarrow_forwardThere are 4 classes of biochemical families (Carbohydrates, Proteins, Lipids, and Nucleic Acids). Identify the biochemical family that is described by the statements below. 1. The monomer of this biochemical family is called an amino acid. 2. DNA is an example of this biochemical family. 3. Glucose and sucrose are an example of this biochemical family. 4. Vitamin A, steroids, waxes, and phospholipids are all examples of this biochemical family. 5. This diverse biochemical family is known for being nonpolar and insoluble in water. 6. This biochemical family is the genetic material in an organism and is made up of a phosphate, sugar, and base monomer. 7. The monomer of this biochemical family contains an amine and a carboxylic acid functional group. Two monomers are linked together through condensation polymerization of these two functional groups.arrow_forward
- A protein has been sequenced after cleavage of disulfide bonds. The protein is known to contain 3 Cys residues, located as shown here. Only one of the Cys has a free —SH group, and the other two are involved in an —S—S— bond. The only methionine and the only aromatic amino acid (Phe) in this protein are in the positions indicated. Cleavage of the intact protein (i.e., withdisulfide bonds intact) by either cyanogen bromide or chymotrypsin does not break the protein into two peptides. Where is the —S—S— bond (i.e., AB, BC, or AC)?arrow_forwardImagine that you were asked to denature a protein; you know you can do so using urea. Your protein binds ATP (a nucleotide). You decide to add ATP to your denatured protein:urea mixture and then add water to dilute the urea and facilitate refolding of the protein. How do you expect the addition of the ATP will affect the free energy of refolding? Consider Gibbs free energy.arrow_forwardCan I get a visual (drawn on paper or on a software; like chem draw or chem sketch)? Illustrate detailed chemical scheme for how you will functionalize your biomolecule to the surface of the sensor (e.g. if you wanted to functionalize a protein to a gold SPR chip, you should could show the assembly of an amine-terminated SAM, followed by carbodiimide coupling of. the protein). These schemes should be drawn using a chemical illustration software package (ChemDraw / ChemSketch).Your chemical schemes should be as detailed as necessary to accurately depict the chemical design of your system, DO NOT INCLUDE a step-wise mechanism, it is not necessary. You should include at least one literature reference to support your conjugation strategy.arrow_forward
- The E. coli enzyme triose phosphate isomerase has a pI of 5.7. What would the charge on this enzyme be below a pH of 5.7? What about above? What would the charge be at a pH of 5.7?arrow_forwarddetail how cation exchange chromatography works and what you would use to elute your target protein. What protein information would you need to facilitate this approach? Would you need to do any protein engineering to utilize cation exchange chromatography, justify your answer?arrow_forwardWhat happens when an enzyme is denatured? Can a denatured enzyme be "re-natured"? Explain.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON