
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
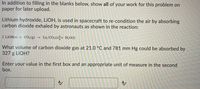
Transcribed Image Text:In addition to filling in the blanks below, show all of your work for this problem on
paper for later upload.
Lithium hydroxide, LİOH, is used in spacecraft to re-condition the air by absorbing
carbon dioxide exhaled by astronauts as shown in the reaction:
2 LIOH(s) + CO2(g) →
Liz CO3(s)[+ H2O(1)
What volume of carbon dioxide gas at 21.0 °C and 781 mm Hg could be absorbed by
327 g LIOH?
Enter your value in the first box and an appropriate unit of measure in the second
box.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the following chemical reaction: 2Al(s) + 3H₂SO4 (aq) → Al₂(SO4)3 (aq) + 3H₂ (g) A student collected 316 mL of hydrogen gas over water at a temperature of 29.0 °C and an atmospheric (total) pressure of 771 torr. The vapor pressure of water at 29.0 °C is 30.0 torr. Calculate the mass (g) of aluminum metal required to produce the hydrogen gas.arrow_forwardAn experiment is performed to determine the volume of gas produced by the following reaction: 2HCl(aq) + Mg(s) MgCl2(aq) + H2(g) A. What is the maximum mass of Mg in g that could be reacted at 20oC temperature and 1.0atm, if the volume of the gas burette is 50mL? (Hint: Find moles of H2 using the ideal gas law; then, convert to g of Mg.) B. If the experiment were moved to a higher elevation and the pressure was decreased to 0.95 atm would the maximum amount of Mg increase or decrease? C. If 16mg of Mg were reacted, what is the volume of gas produced in this reaction? D. What is the solvent for this reaction? E. Would the HCl(aq) be considered a strong, weak, or non-electrolyte? F. Would the MgCl2(aq) be considered a strong, weak, or non-electrolyte?arrow_forwardCalculate the volume occupied by 25.8 g of methane gas (CH4) at 28°C and 1.0 atm (R = 0.0821 L·atm·K-1·mol-1). 0.039 L 8.7 L 39.8 L 99.2 L 59.7 Larrow_forward
- A 10.0 L sample of CO2 gas at 25.0 degrees celsius is heated at a constant pressure until the volume has doubled. What is the final ideal temperature of CO2 in degrees celsius?arrow_forwardPlease don't provide handwritten solution ....arrow_forwardSulfuric acid is essential to dozens of important industries from steelmaking to plastics and pharmaceuticals. More sulfuric acid is made than any other industrial chemical, and world production exceeds 2.0 × 10¹¹ kg per year. The first step in the synthesis of sulfuric acid is usually burning solid sulfur to make sulfur dioxide gas. Suppose an engineer studying this reaction introduces 3.1 kg of solid sulfur and 10.0 atm of oxygen gas at 800. °C into an evacuated 70.0 L tank. The engineer believes K = 0.98 for the reaction at this temperature. Calculate the mass of solid sulfur he expects to be consumed when the reaction reaches equilibrium. Round your answer to 2 significant digits. Note for advanced students: the engineer may be mistaken in his belief about the value of K, and the consumption of sulfur you calculate may not be what he actually observes. p' kg ☐ x10 ×arrow_forward
- When collecting a gas over water it is necessary to correct for the vapor pressure of water in order to determine the pressure of the gas you are collecting. We will do this by using a graph of ln P in mmHg versus 1/T where T is the temperature in Kelvin. The equation is y = -5206.4 x + 20.621 (With an R^2 = 0.9999). If you collect a gas at 26.8 ℃, what is the value of “x” that you want to use in the equation. (Keep the answer for the next question). (Keep extra digits, you do not want to round in the middle of this calculation). Select one: a. 0.003660992 b. 0.037313433 c. 0.003378378 d. 299.95 e. 0.0273150 f. 273.15 g. None of these h. 0.003333889 i. 0.0027315arrow_forwardFor many purposes we can treat ammonia (NH, ) as an ideal gas at temperatures above its boiling point of -33. °C. Suppose the temperature of a sample of ammonia gas is lowered from 13.0 °C to –16.0 °C, and at the same time the pressure is increased by 10.0%. increase x10 Does the volume of the sample increase, decrease, or stay the same? decrease ? stays the same If you said the volume increases or decreases, calculate the percentage change in % the volume. Round your answer to the nearest percent.arrow_forwardPV=nRT R=0.0821 atm•L/mol•K °C + 273.15 = K 1 atm = 760 mmHg Many metals produce hydrogen gas when dropped in water. For example: Ca (s) +2 H20 (1) → Ca(OH)2 (aq) + H2(g) How much hydrogen gas (in liters) would be produced from dropping 5.0 grams of Calcium into an excess of water, at 25°C and 755 mmHg?arrow_forward
- Below is the equation for the combustion of methane (CH ). How many liters of HO are formed by the complete combustion of 1.8 grams of methane at 25 °C and 1.0 atm pressure? CH +20, + CO+2 H,0 → '4(g) - 2/g) (g)arrow_forwardOxygen gas can be prepared by heating potassium chlorate according to the following equation: 2KCIO3(s)2KCI(s) + 302(g) The product gas, O2, is collected over water at a temperature of 25 °C and a pressure of 758 mm Hg. If the wet O2 gas formed occupies a volume of 8.11 L, the number of grams of O, formed is g. The vapor pressure of water is 23.8 mm Hg at 25 °C.arrow_forwardWhat is the temperature of 100.0 g of chlorine gas (molar mass = 70.90 g/mol) in a 4.00 L container at 1.0 atm? R= 0.082 L atm/mol Karrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY