
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

Transcribed Image Text:Here's a hint!
Identify the substances oxidized and reduced.
Standard Electrode Potentials (298 K)
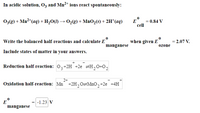
Transcribed Image Text:In acidic solution, Oz and Mn²+ ions react spontaneously:
03(g) + Mn²*(aq) + H,O(1) → 0,(8) + MnO,(s) + 2H*(aq)
E
cell
0.84 V
Write the balanced half-reactions and calculate E
when given E
= 2.07 V.
manganese
ozone
Include states of matter in your answers.
Reduction half-reaction: 0,+2H +2e =H,0+02
2+
Oxidation half-reaction: Mn +2H,0=MnO,+2e +4H
E
-1.23 V
manganese
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the galvanic cell based on the following half-reactions. Ag+ + e− → Ag ℰ° = +0.80 V Fe2+ + 2 e− → Fe ℰ° = −0.44 V (a) Determine the overall cell reaction and calculate ℰ ° cell . (b) Calculate ΔG° and K for the cell reaction at 25°C. (c) Calculate ℰcell at 25°C when [Ag+ ] = 1.0 ✕ 10−4 M and [Fe2+ ] = 1.0 ✕ 10−2 M.arrow_forwardA galvanic cell is powered by the following redox reaction: 3 Br₂(1) + 2NO(g) + 4H₂O(1) - 6 Br (aq) + 2NO3(aq) + 8H (aq) Answer the following questions about this cell. If you need any electrochemical data, be sure you get it from the ALEKS Data tab. Write a balanced equation for the half-reaction that takes place at the cathode. Write a balanced equation for the half-reaction that takes place at the anode. Calculate the cell voltage under standard conditions. Round your answer to 2 decimal places. 0 0 E = v 00 Śarrow_forwardConstants The following values may be useful when solving this tutorial. Constant Cu Eco R F T Value 0.337 V -0.277 V 8.314 J. mol-¹.K-¹ 96,485 C/mol 298 Karrow_forward
- Electrolysis of molten NaClNaCl is done in a Downs cell operating at 7.0 volts (V) and 5.0×104 AA . How much Na(s)Na(s) and Cl2Cl2 can be produced in eight hours in such a cell? Enter the mass of NaNa and mass of Cl2Cl2 in grams, separated by a comma.arrow_forwardAlkaline Batteries (AA/AAA) Cathode: 2 Mno2 (s) + H2O (I) + 2 e→ Mn,O3 (s) + 2 OH: (aq) +3 · Anode: Zn (s) + 2 OH (aq) → ZnO (s) + H2O (I) + 2 e- · Anode: Zn; Cathode: MnO2 (s) What is the overall balanced equation for the redox reaction occurring in an alkaline (AA/AAA) battery?arrow_forwardGalvanic cell has 2 half reactions O2(g) + 4H+(aq) + 4e- → 2H2O(l) E0red = +1.23 V Cr3+(aq) + 3e- → Cr(s) E0red = -0.74 V Write a balanced equation that describes the cell, ensuring that E0cell > 0 V and Calculate E0cellarrow_forward
- Consider the standard reduction potentials and redox couples used in a galvanic cell. Unbalanced Half-Reaction E°(V) MnO4 (aq) + 3e MnO2(s) (at basic pH) 0.59 Fe3+(aq) + e- Fe2*(aq) 0.77 What is the balanced reaction taking place in the cell (in basic solution)? 2 H20(1) + Mn04 (aq) + 3 Fe3+(aq) → 3 Fe2*(aq) + MnO2(s) + 4 OH- MnO2(s) + 4 OH¯ + 3 Fe3+(aq) 3 Fe2+(aq) + 2 H20(1) + MnO4¯(aq) MO 3 Fe2*(aq) + 2 H2O(1) + MnO4¯(aq) MnO2(s) + 4 OH¯ + 3 Fe3+(aq) M 3 Fe2+(aq) + MnO2(s) + 4 OH → 2 H20(1) + MnO4 (aq) + 3 Fe3+(aq)arrow_forwardConsider the galvanic cell based on the following half-reactions. Ag+ + e Ni²+ + 2e →→ Ni (a) Determine the overall cell reaction and calculate Omit states-of-matter from your answer.) chemPad XX→ (c) 8 cell → Ag 8⁰ = +0.80 V 8° -0.23 V Calculate 8 Help Greek º (b) Calculate AGº (in kJ) and K for the cell reaction at 25°C. AGO kJ K (in V). (Use the lowest possible whole number coefficients. cell (in V) at 25°C when [Ag+] = 1.0x10-4 M and [Ni²+] = 1.0×10-² M. cell Varrow_forwardG ロ・ロ A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: 2+ 3+ MnO2 (s) + 4H* (aq) +2Cr²* (aq) → Mn²* (aq) + 2H2O (1) +2Cr³+ (aq) Suppose the cell is prepared with 0.190 M H* and 6.41 M Cr²+ in one half-cell and 0.205 M Mn2+ and 1.94 M Cr3+ in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits. ☐ ☐ x10 μarrow_forward
- A galvanic cell is powered by the following redox reaction: 2 Br,(1) + N,H,(aq) + 4OH (aq) 4 Br (aq) + N,(g) + 4 H,O(1) Answer the following questions about this cell. If you need any electrochemical data, be sure you get it from the ALEKS Data tab. Write a balanced equation for the half-reaction that takes place at the cathode. ローロ e Write a balanced equation for the half-reaction that takes place at the anode. Calculate the cell voltage under standard conditions. E - Round your answer to 2 decimal places. のarrow_forwarda) Balance the following redox reaction in basic solution: H2O2(aq) + ClO2(aq) → ClO2-(aq) + O2(g) b) Sketch the voltaic cell for the redox reaction, labeling the direction for electron flow, the anode and cathode , and the species present in each half-cell. Show all the work please!arrow_forwardWhat is the voltage of the following cell? Use the Reduction Potential Table found in Chapter 17 notes. Cr(s)|Cr³*(0.25 M) || |2(s)|1-(0.20 M)|Pt(s)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY