
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
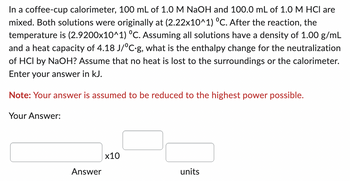
Transcribed Image Text:In a coffee-cup calorimeter, 100 mL of 1.0 M NaOH and 100.0 mL of 1.0 M HCI are
mixed. Both solutions were originally at (2.22x10^1) °C. After the reaction, the
temperature is (2.9200x10^1) °C. Assuming all solutions have a density of 1.00 g/mL
and a heat capacity of 4.18 J/°C.g, what is the enthalpy change for the neutralization
of HCI by NaOH? Assume that no heat is lost to the surroundings or the calorimeter.
Enter your answer in kJ.
Note: Your answer is assumed to be reduced to the highest power possible.
Your Answer:
Answer
x10
units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A 5.71 g sample of an unknown salt (MM = 116.82 g/mol) is dissolved in 150.00 g water in a coffee cup calorimeter. Before placing the sample in the water, the temperature of the salt and water is 23.72 °C. After the salt has completely dissolved, the temperature of the solution is 28.54 °C. What is the total mass inside the calorimeter in grams?arrow_forwardYou are performing an important biological experiment which requires that you set up a water bath at 37.0 oC (physiological temperature). You add cold tap water at 20.0 oC, and hot tap water at 55.0 oC. You start with 85.0 g cold water, what mass of hot water must be added to get to 37.0 oC? (Specific heat capacity of water is 4.184 J/g.K)arrow_forward100.0 ml of 0.115 m potassium hydroxide solution is mixed with 75.0 ml of 0.245 M nitric acid solution in a coffee cup calorimeter. Before mixing, both solutions are initially at 23.85 C;after mixing, the temperature of the mixture in the coffee cup calorimeter increases to 26.30 C. Determine the enthalpy change(delta h) of reaction in kJ/mol for the reaction as written below. KOH(aq) + HNO3(aq) ⮕ KNO3(aq) + H2O(aq) Assume the resulting solution density is 1.03 g/mL. Specific heat of solution=4.20 J/g Carrow_forward
- A 5.71 g sample of an unknown salt (MM = 116.82 g/mol) is dissolved in 150.00 g water in a coffee cup calorimeter. Before placing the sample in the water, the temperature of the salt and water is 23.72 °C. After the salt has completely dissolved, the temperature of the solution is 28.54 °C. Was the dissolution process endothermic or exothermic?arrow_forward22.214 g of a metal at 99.73°C was added to 14.2 g of water at 19.05°C. The temperature of the mixture rose to 28.78°C. What is the specific heat of the metal? SH2O = 4.184 J/g°Carrow_forwardWhen 6.54 grams of Zn is placed in 500.0 mL of 1.00 M CuSO4(aq) in a coffee cup calorimeter, it reacts completely to displace copper. The temperature of the solution rises from 20.0˚C to 30.4˚C. Assume the coffee cup itself gains no heat and that the solution has the same density (1.00 g/mL) and specific heat (4.184 J/g˚C) as pure water. (a) How much heat does the solution gain during this reaction? (in J)arrow_forward
- A 88.8 g piece of aluminum (which has a molar heat capacity of 24.03 J/°C·mol) is heated to 82.4°C and dropped into a calorimeter containing water (specific heat capacity of water is 4.18 J/g°C) initially at 22.3°C. The final temperature of the water is 24.8°C. Ignoring significant figures, calculate the mass of water in the calorimeter.arrow_forward[11] A 10.00 g sample of a metal alloy was heated to 88.99 °C. It is then quickly dropped into 40.0 g of water in a calorimeter. The water temperature rises from 19.73 °C to 24.23 °C. Calculate the specific heat of the alloy. [Specific Heat of Water = 4.184 J/g °C and Heat Capacity of Calorimeter = 12.6 J/°C]arrow_forwardIn a coffee-cup calorimeter, 59.0 mL of 0.100 M AgNO3 and 59.0 mL of 0.100 M HCI are mixed to yield the following reaction. Ag (aq) + Cl(aq) → AgCl(s) The two solutions were initially at 19.10°C, and the final temperature is 19.90°C. Calculate the heat that accompanies this reaction in kJ/mol of AgCl formed. Assume that the combined solution has a mass of 118.0 g and has a specific heat capacity of 4.18 1/°C 9- 407 kJ/molarrow_forward
- In a coffee cup calorimeter with a heat capacity of 21.5 J/ºC, 225 mL of 0.20 M KOH at 22.3 ºC neutralizes 225 mL of 0.20 M HCl at 22.3 ºC. After the reaction occurs, the temperature of the resulting mixture is 29.2 ºC. The density of the final solution is 1.00 g/mL and the specific heat is 4.18 J/g°C. Calculate the Heat of neutralization of KOH.arrow_forwardIn a constant-pressure calorimeter, 65.0 mL of 0.910 M H₂SO, was added to 65.0 mL of 0.300 M NaOH. The reaction caused the temperature of the solution to rise from 24.00 °C to 26.04 *C. If the solution has the same density and specific heat as water (1.00 g/mL and 4.184 J/g °C), respectively), what is AH for this reaction (per mole of H₂O produced)? Assume that the total volume is the sum of the individual volumes. AH = V 5 R B 6 MacBook Air N 39 8 M command P option kJ/mol H₂Oarrow_forwardwhen 50.0 mL of 0.200 M NaOH is mixed with 50 mL of 0.200 M HCl in a coffee cup calorimeter, the temperature increases from 23.5 degrees C to 26.5 degrees C. Assuming that the density of the resulting solution is 1.00 g/mL and the specific heat capacity of the solution is 4.18 J/g degrees C, what is qrxn?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY