
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
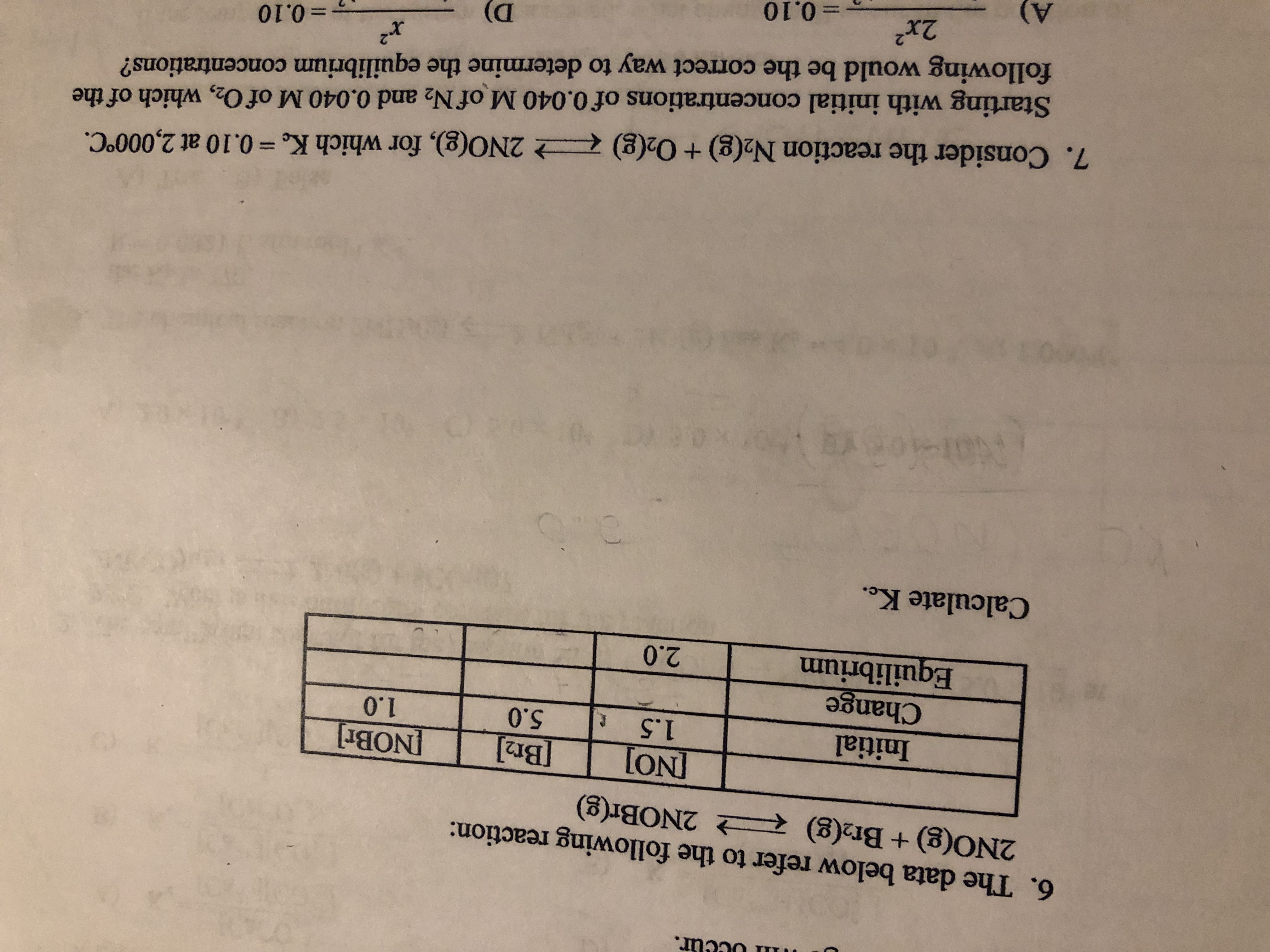
Transcribed Image Text:il occur.
6. The data below refer to the following reaction:
2N0(g) + Br2(g) R
之2NOBr(g)
Initial
Change
uilibri
1.5 5.0
1.0
Equlbrium20
Calculate Ke.
7. Consider the reaction N2(g) + 02(g)-그 2N0(g), for which K. = 0.10 at 2,000°C.
Starting with initial concentrations of 0.040 M of N2 and 0.040 M of O2, which of the
following would be the correct way to determine the equilibrium concentrations?
2
=0.10
2x2
D)
2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 4 images

Knowledge Booster
Similar questions
- Consider the following system at equilibrium where AH° = -111 kJ, and K = 0.159, at 723 K. %3D %3D N2(g) + 3H2(g) =2NH3(g) When 0.22 moles of H2(g) are added to the equilibrium system at constant temperature: The value of Kc The value of Qc. Kc. The reaction must O run in the forward direction to restablish equilibrium. O run in the reverse direction to restablish equilibrium. O remain the same. It is already at equilibrium. The concentration of N2 will Submit Answer Retry Entire increase. roup attempts remaining decrease. remain the same.arrow_forwarduju ts Search G Free Online Survey... Submit Answer Home - Academia... H Modules Retry Entire Group Consider the following reaction where K = 6.50x10-3 at 298 K: 2 NOBr (g) 2 NO(g) + Br₂ (g) A reaction mixture was found to contain 8.48x10-2 moles of NOBr (g), 4.39x10-2 moles of NO (g), and 4.04x10-2 moles of Br₂ (g), in a 1.00 liter container. Indicate True (T) or False (F) for each of the following: 1. In order to reach equilibrium NOBr(g) must be consumed. 2. In order to reach equilibrium K must increase. 3. In order to reach equilibrium NO must be produced. 4. Q is less than K. 5. The reaction is at equilibrium. No further reaction will occur. 8 Use the References to access important values. f needed for this question. Create Your Rubric-... [References] K 8 more group attempts remaining 9 The Utility Experts ... PPT 4:33 PM 12/15/2023 prt sc backspacearrow_forwardConsider this equilibrium reaction at 400 K. Br, (g) + Cl,(g) = 2 BICI(g) K. = 7.0 If the composition of the reaction mixture at 400 K is [BrCI] = 0.00248 M, [Br,] = 0.00570 M, and [Cl,] = 0.00105 M, what is the reaction quotient, Q? Q = How is the reaction quotient related to the equilibrium constant, Kç, for this reaction? Oe K. Oe = K.arrow_forward
- Consider the following system at equilibrium where K. 7.00x10-5 and AH° = 182 kJ/mol at 673 K. NH4I (s) = NH3 (g) + HI (g) The production of NH3 (g) is favored by: Indicate True (I) or False (F) for each of the following: 1. increasing the temperature. v 2. decreasing the pressure (by changing the volume). v 3. increasing the volume. v 4. adding NH4I . 5. removing HI .arrow_forward9 q eq eq req req 1req OWLv2 | Online teaching and learning resource from Cengage Le... Submit Answer [Review Topics] [References] Use the References to access important values if needed for this question. The equilibrium constant, Kp, for the following reaction is 0.497 at 500. K. PC15 (9) PC13 (g) + Cl₂ (g) If an equilibrium mixture of the three gases in a 12.7 L container at 500. K contains PC15 at a pressure of 0.804 atm and PC13 at a pressure of 0.911 atm, the equilibrium partial pressure of Cl₂ is l atm. b Home | bartleby Retry Entire Group 9 more group attempts remainingarrow_forwardConsider the following system at equilibrium where K. = 9.52x102 and AH° = 18.8 kJ/mol at 350 K. CH4 (g) + CC14 (g) =2 CH,Ch (g) The production of CH,Cl2 (g) is favored by: Indicate True (T) or False (F) for each of the following: |1. decreasing the temperature. | 2. increasing the pressure (by changing the volume). | 3. decreasing the volume. v 4. adding CH,Cl . | 5. removing CCI4 .arrow_forward
- ■ W Chapter... G N. O LCG a Ⓒ BA H H The initial concentrations or pressures of reactants and products are given for each of the following systems. Calculate the reaction quotient and determine the direction in which each system will proceed to reach equilibrium. (The system is considered in equilibrium if K, and Q are within 5% of each other.) (a) 2 NH3(g) = N₂(g) + 3 H₂(g) [NH3] = 0.485 M, [N₂]; = 0.160 M, [H₂] = 0.110 M, K = 18 What is the reaction quotient? What direction will the reaction shift to? O To the left, i.e. the reactant-side. O To the right, i.e. the product-side. O It will not shift. (b) 2 NH3(9) N₂(g) + 3 H₂(g) What is the reaction quotient? What direction will the reaction shift to? O To the left, i.e. the reactant-side. P (NH3) = 1.90 atm, P(N₂) = 11.65 atm, P(H₂) = 11.65 atm, K = 6.6x104 O To the right, i.e. the product-side. O It will not shift. (c) 2 SO 3(g) 2 SO₂(g) + O₂(9) What is the reaction quotient? webassign.net [SO3] = 1.65 M, [SO₂), = 1.65 M, [0₂], =…arrow_forwardFor the reaction 2CH,(g) =C,H2(g) + 3H2(g) Ke = 0.155 at 1611 °C. What is K, for the reaction at this temperature? Express your answer numerically. • View Available Hint(s) ? Kp =arrow_forwardCtrl Tab Caps Shift 58°F Sunny Esc Fn Determine the temperature of a reaction if K = 1.20 x 106 when AG° = +21.00 kJ/mol. ! 1 Q A S N FI - - 2 W S F2 X Alt # 3 E Chat D C $ 4 R TI F H O DII % 5 V F5 T G ☀ F6 ^ Question 9 of 15 6 Y B * H & 7 PrtScn U N FB J * 8 Home 1 M ( 9 K End F10 O < 1 0 L PgUp P 4 1 7 +/- : Alt PgDrarrow_forward
- For the equilibrium reaction. 2IBr (g) I2 (g) + Br2 (g) Kc=.0085. If .025 M of IBr is introduced to an empty flask and allowed to reach equilibrium, calculate the final concentrations of all components. Consider the decomposition reaction at 555 K 4POCl3 (g) P4 (g) + 2O2 (g) + 6Cl2 (g) If .450 atm of POCl3 is introduced to an otherwise empty flask and the reaction is allowed to reach equilibrium, the final total pressure is .850 atm. Find Kp and Kc.arrow_forwardient/takeCovalentActivity.do?locator-assignment-take Consider the following system at equilibrium where AH° = 198 kJ, and K. = 2.90x102, at 1.15x10° K. 2S03(g) 2S02(g) + O2(g) If the VOLUME of the equilibrium system is suddenly increased at constant temperature: The value ofK. A. increases. B. decreases. C. remains the same. The value of Q. A. is greater than K.. B. is equal to K. C. is less than K. The reaction must: A. run in the forward direction to reestablish equilibrium. B. run in the reverse direction to reestablish equilibrium. C. remain the same. It is already at equilibrium. The number of moles of O, will: A. increase. B. decrease. C. remain the same. Submit Answer Retry Entire Group 8 more group attempts remaining Prevarrow_forwardq The equilibrium constant, Ke, for the following reaction is 1.80 x 104 at 298 K. [Review Topics] [References] Use the References to access important values if needed for NH4HS(9) NH3(g) + H₂S(9) Calculate the equilibrium concentration of H₂S when 0.322 moles of NH4HS(s) are introduced into a 1.00 L vessel at 298 K. [H₂S] = M Submit Answer Show Hint NOV 30 Le... The equilib tv Retry Entire Group 9 more group attempts remaining Email In Marrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY