Question
thumb_up100%
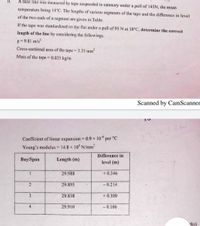
Transcribed Image Text:À base line was measured by tape suspended in catenary under a pull of 145N, the mean
temperature being 14°C. The lengths of various segments of the tape and the difference in level
of the two ends of a segment are given in Table.
If the tape was standardized on the flat under a pull of 95 N at 18°C, determine the correct
length of the line by considering the followings.
g = 9.81 m/s?
Cross-sectional area of the tape = 3.35 mm²
Mass of the tape = 0.025 kg/m
Scanned by CamScanner
Coefficient of linear expansion 0.9 × 10° per °C
%3D
Young's modulus = 14.8 x 10 N/mm
Difference in
Вay'Span
Length (m)
level (m)
29.988
+ 0.346
29.895
- 0.214
3
29.838
+ 0.309
4
29.910
- 0.106
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Similar questions
- An ordinary drinking glass is filled to the brim with 350 mL of water at 100.0 "C. If the temperature is decreased to 20.0 °C, then what volume of water could be added to the glass? The coefficient of volume expansion for glass is 27.0x108 (C)¹. The coefficient of volume expansion for water is 210 x 10-6 (C)1. 'n Gewone drinkglas is tot heelbo gevul met 350 mL water by 100.0 "C. Watter volume water kan by die glas gevoeg word indien die temperatuur tot 20.0 °C daal? Die volume uitsettingskoeffisiënt van glas is 27.0x 10- (C)¹. Die volume uitsettingskoeffisiënt van water is 210 x 108 (C)¹. Select one: O a. 5.12 mL 5.88 mL. Ob. O c. 9.23 mL O d. 0.756 mL Oe. 7.68 mLarrow_forward5 please need help, keep in mind the 7 basic units and watch the sig figs thank you so much.arrow_forwardThe container shown in (Figure 1) is filled with water at a temperature of 15 °C and a depth of h = 1.6 m. Figure 1 m h < 1 of 1 Part A If the container has a mass of 45 kg, determine the combined weight of the container and the water. Express your answer to three significant figures and include the appropriate units. WT- = Submit O μᾶ Value Provide Feedback Request Answer O kN ? 1arrow_forward
- A hot air balloon uses the principle of buoyancy to create lift. By making the air inside the balloon less dense then the surrounding air, the balloon is able to lift objects many times its own weight. A large hot air balloon has a maximum balloon volume of 2090 m3. a. If the air temperature in the balloon is 54 °C, how much additional mass, in kilograms, can the balloon lift? Assume the molar mass of air is 28.97 g/mol, the air density is 1.20 kg/m3, and the air pressure is 1 atm.arrow_forwardNeeds Complete typed solution with 100 % accuracy.arrow_forward
arrow_back_ios
arrow_forward_ios