
Physics for Scientists and Engineers: Foundations and Connections
1st Edition
ISBN: 9781133939146
Author: Katz, Debora M.
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
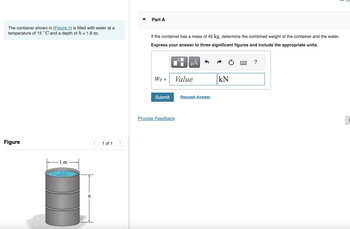
Transcribed Image Text:The container shown in (Figure 1) is filled with water at a
temperature of 15 °C and a depth of h = 1.6 m.
Figure
1 m
h
<
1 of 1
Part A
If the container has a mass of 45 kg, determine the combined weight of the container and the water.
Express your answer to three significant figures and include the appropriate units.
WT- =
Submit
O
μᾶ
Value
Provide Feedback
Request Answer
O
kN
?
1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 7 images

Knowledge Booster
Similar questions
- a 25cm diameter cylinder that is 36cm long contains 60g of oxygen gas at 20 degree c what is the number density of the oxygen? (express in molecules per meter cubed) what is the reading of a pressure gauge attached to the tank? (express with appropriate units)arrow_forwardIn a hypothetical diesel engine the fuel is compressed from 528mL to 39.7 mL. This rasies the temperature of the fuel form 25°C to its ignition temperature of 210°C. If the fuel begins at 1.00atm, what is the pressure of the fuel at its ignition temperature.? Express your answer to 1 decimal place. Be sure to use the proper abbreviation of the UNITSarrow_forwardAir flows at 210 m/s through the pipe. The temperature is 400 K and the absolute stagnation pressure is 280 kPa. Assume isentropic flow. For air R = 286.9 J/[kg - K] and k=1.40. (Figure 1) Figure 0.3 marrow_forward
- Presents the diagram of the problem, necessary formulas, clearance and numerical solution: On June 26, the US Brookhaven National Laboratory (BNL) announced that the Guinness World Records (GWR) organization had granted him the temperature record highest ever achieved: gold ion collisions at nearly the speed of light at the accelerator of Relativistic Heavy Ion Collider particles had formed a plasma at 4 billion degrees Celsius. Convert this value to ◦C and K.arrow_forwardNeeds Complete typed solution with 100 % accuracy.arrow_forwardLet's say you park your car outside overnight during the winter, when you first parked your car the temperature was 29.0 degrees C but overnight the temperature drops to -7.0 degrees C. If your initial gauge pressure was 200kPa, what will be the gauge pressure (in KPa) after the change in temperature?arrow_forward
- This was wrong. Can you solve this again with these numbers? What is the root mean square velocity, vrms, for Hydrogen molecules (H2) at 20oC? Hint: How many amu does an H2 molecule contain. 1 amu = 1.67 x 10-27 kg Boltzman's Constant, k = 1.38 x 10-23 J/K Give your answer in m/s to 4 significant figures (NO DECIMALS)arrow_forwardpls help ty!arrow_forwardCalculate the average density of the following astronomical body: Jupiter. Where does the value fit among those listed in the table below? Look up the density of a typical rock, such as granite in another source and compare the density of Jupiter to it. Table: Densities of Some Common Substances at Standard Temperature (0°C) and Pressure (Atmospheric) Substance ρ (kg/m3) Substance ρ (kg/m3) Air 1.29 Ice 0.917 ✕ 103 Aluminum 2.70 ✕ 103 Iron 7.86 ✕ 103 Benzene 0.879 ✕ 103 Lead 11.3 ✕ 103 Copper 8.92 ✕ 103 Mercury 13.6 ✕ 103 Ethyl Alcohol 0.806 ✕ 103 Oak 0.710 ✕ 103 Fresh Water 1.00 ✕ 103 Oxygen gas 1.43 Glycerin 1.26 ✕ 103 Pine 0.373 ✕ 103 Gold 19.3 ✕ 103 Platinum 21.4 ✕ 103 Helium gas 1.79 ✕ 10−1 Sea Water 1.03 ✕ 103 Hydrogen gas 8.99 ✕ 10−2 Silver 10.5 ✕ 103arrow_forward
- The vapor pressure of water at 25°C is 0.0313 atm. Calculate the vapor pressure in kPa.Round answer to 3 significant digits.arrow_forward1.21 A team is designing a helium-filled balloon that will fly to an altitude of 80,000 ft. As the balloon ascends, the upward force (buoyant force) will need to exceed the total weight. Thus, weight is critical. Estimate the weight (in newtons) of the helium inside the balloon. The balloon is inflated at a site where the atmospheric pressure is 0.89 bar and the temperature is 22°C. When inflated prior to launch, the balloon is spherical (radius 1.3 m) and the inflation pressure equals the local atmospheric pressure.arrow_forwardInvalid path. A light balloon is filled with 383 m3 of helium at atmospheric pressure. (a) At 0°C, the balloon can lift a payload of what mass? kg (b) In the table below, observe that the density of hydrogen is nearly half the density of helium. What load can the balloon lift if filled with hydrogen? kg Densities of Some Common Substances at Standard Temperature (0°C) and Pressure (Atmospheric) p(kg/m3) p(kg/m3) Substance Substance Air 1.29 Ice 0.917 X 103 7.86 X 103 11.3 X 10 13.6 X 10 Aluminum 2.70 X 103 Iron Benzene 0.879 X 103 Lead 8.92 X 103 Mercury Copper Ethyl alcohol 0.806 X 105 Oak 0.710 X 103 Fresh water 1.00 X 103 Oxygen gas Pine 1.43 Glycerin 1.26 X 103 0.373 X 103 Gold 19.3 X 10 Platinum 21.4 X 10 1.03 X 10 10.5 X 10 Helium gas 1.79 X 10-1 Seawater Hydrogen gas 8.99 X 10-2 Silverarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning
