
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
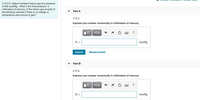
Transcribed Image Text:A 10.5 L balloon contains helium gas at a pressure
of 655 mmHg . What is the final
millimeters of mercury, of the helium gas at each of
the following volumes if there is no change in
temperature and amount of gas?
pressure,
in
Part A
17.0 L
Express your answer numerically in millimeters of mercury.
ΑΣφ
?
mmHg
Submit
Request Answer
Part B
2.70 L
Express your answer numerically in millimeters of mercury.
V ΑΣΦ
?
P2 =
mmHg
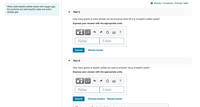
Transcribed Image Text:I Review I Constants I Periodic Table
When solid lead(II) sulfide reacts with oxygen gas,
the products are solid lead(II) oxide and sulfur
dioxide gas.
Part C
How many grams of sulfur dioxide can be produced when 64.0 g of lead(II) sulfide reacts?
Express your answer with the appropriate units.
HÁ
?
Value
Units
Submit
Request Answer
Part D
How many grams of lead(II) sulfide are used to produce 125 g of lead(II) oxide?
Express your answer with the appropriate units.
µA
?
Value
Units
Submit
Previous Answers Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the following reaction, 8.68 grams of nitrogen gas are allowed to react with 5.94 grams of oxygen gas.nitrogen (g) + oxygen (g) nitrogen monoxide (g)What is the maximum amount of nitrogen monoxide that can be formed? ________ grams What is the FORMULA for the limiting reagent? ______ What amount of the excess reagent remains after the reaction is complete? _______arrow_forwardUnanswered Submit T14HW Question 16 Homework Unanswered Magnesium oxide and ammonium bromide react to produce ammonia gas, NH3, water, and magnesium bromide. If 152 g of magnesium oxide are reacted with 595 g of ammonium bromide, 74.2 g of ammonia are produced. What is the percent yield for this reaction? Type your numeric answer and submit Submit Unanswered Fullscreerarrow_forwardAqueous hydrobromic acid (HBr) will react with solid sodium hydroxide (NaOH) to produce aqueous sodium bromide (NaBr) and liquid water (H,O). Suppose 14.6 g of hydrobromic acid is mixed with 11. g of sodium hydroxide. Calculate the maximum mass of water that could be produced by the chemical reaction. Round your answer to 3 significant digits. olo Ar Explanation Check © 2022 McGraw Hill LLC. AIL Rights Reserved. Terms of Use | Privacy Center | Accessibility ................... ..arrow_forward
- If 11.8-g of hydrogen gas, H,, reacts wiwth 14.2-g of nitrogen gas, N2, what is the maximum amount of ammonia gas, NH, that can be produced? 66.7-g 17.3-g 8.64-g 33.2-g What was the limiting reactant in question number 16? N2 H2 Neither: they were in perfect amountsarrow_forwardPlease don't provide handwriting solutionarrow_forwardUse the References to access important values if needed for this question. For the following reaction, 3.56 grams of nitrogen gas are mixed with excess hydrogen gas. The reaction yields 3.75 grams of ammonia. nitrogen (g) + hydrogen (g) ammonia(g) grams What is the theoretical yield of ammonia ? What is the percent yield for this reaction ? Submit Answer 5 question attempts remaining %arrow_forward
- The theoretical yield of a reaction is the amount of product obtained if the limiting reactant is completely converted to product. Consider the reaction: 2 Fe(s) + O2(g) → 2 FeO(s) If 9.250 g Fe is mixed with 13.39 g O2, calculate the theoretical yield (g) of FeO produced by the reaction. Submit Show Approach Show Tutor Stepsarrow_forwardPls help me solve this question, explain and make sure everything is correct 1000% thanksarrow_forwardI need help with this chemistry equation: Barium chloride and sodium sulfate react according to the equation below. Answer the following questions about this reaction.BaCl2 + Na2SO4 → BaSO4 + 2NaClHow many grams of barium chloride are needed to make 100 grams of barium sulfate, assuming that the reaction yield is 100%? Answer format: 52.2 g __________________arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY