
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
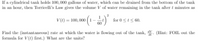
Transcribed Image Text:If a cylindrical tank holds 100,000 gallons of water, which can be drained from the bottom of the tank
in an hour, then Torricelli's Law gives the volume V of water remaining in the tank after t minutes as
1
V (t) = 100, 000 (1-
60
for 0 <l< 60.
Find the (instantancous) rate at which the water is flowing out of the tank, V. (Hint: FOIL out the
formula for V(L) first.) What are the units?
IP
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hot gaes Expanding A mixture hot gases expands, changing volume from 2.45 L to 6.82 L, against a constant pressure of 3.22 atm. The P-Vwork involved is Latm .arrow_forwardUsing (a) the ideal gas law and (b) the van der Waals equation, calculate the pressure exerted by 50.0 g carbon dioxide in a 1.00-L vessel at 25°C. Do attractive or repulsiveforces dominate?arrow_forwardCalculate the most probable speed of oxygen (O2) molecules in a gas at T = 334 K, O2 atomic mass is 32 amu, where the atomic mass unit (amu) is 1.66 x 10-27 kg. Provide your answer in units of meters per second, but do not include the units in your answer, just the number in normal form to 3 significant digitsarrow_forward
- The compressibility factor Z = PV/nRT for O2 at -38.6°C and 519.6 atm is 1.60; at 162.8°C and 105.4 atm, it is 0.99. A certain mass of oxygen occupied a volume of 2.10 L at 162.8°C and 105.4 atm. Calculate the volume occupied by the same quantity of oxygen at -38.6°C and 519.6 atm. place the answer rounded to the hundredths place Please provide only typed answer solution no handwritten solution needed allowedarrow_forwardAt 298K, N2 molecules have a mean speed of 475 m/s. As a consequence, the probability to randomly pick 1 molecule at 475.00 m/s is extremely high. Select one: True Falsearrow_forwardIf the partial pressure of N2 is 2.70 x 105 Pa and the partial pressure of Ne is 5.00 x 105 Pa in a given mixture, what is the mole fraction of Ne (XNe)?arrow_forward
- High-pressure liquid chromatography (HPLC) is a method used in chemistry and biochemistry to purify chemical substances. The pressures used in this procedure range from around 500 kilopascals (500,000 Pa) to about 60,000 kPa (60,000,000 Pa). It is often convenient to know the pressure in torr. If an HPLC procedure is running at a pressure of 1.45x107 Pa, what is its running pressure in torr? Express the pressure numerically in torr. ► View Available Hint(s) 1.45x107 Pa = VE ΑΣΦ ? torrarrow_forwardSince the specific volume of nitrous oxide gas (N 2 O) at a pressure of 100 kPa and a temperature of 47 ° C multiplied by the compressibility factor is 0.453 m ^ 3 / kg, what is the compressibility factor of this gas?arrow_forwardPlease don't provide handwritten solution .....arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY