
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all working!!!!!!!
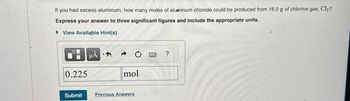
Transcribed Image Text:If you had excess aluminum, how many moles of aluminum chloride could be produced from 16.0 g of chlorine gas, Cl₂?
Express your answer to three significant figures and include the appropriate units.
▸ View Available Hint(s)
0.225
HA
mol
?
Submit
Previous Answers
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- We want to inform you that your performance as a tutor is being evaluated and will be reported to the administration. Additionally, there will be an online ranking system that not only assesses the overall performance of Bartleby as a website but also ranks individual tutors based on their question-and-answer accurate contributions. This ranking will be shared with management. Please ensure that your responses are of the highest quality, as multiple requests may be directed to the same person for assessment.arrow_forwardques 2 part carrow_forwardThis is NOT graded!arrow_forward
- Organic Chem 1 Week 5 Lab- Student.docx - Saved to this PC P Search shiku24 Design Layout References Mailings Review View Developer Help A A Aa v Ao E - E MaBbCcDd AaBbCc Dd AaBbCc AaBbCcD AaBi 1 Normal 1 No Spac. Heading 1 * A. P. A 三。、。 Reola Heading 2 T'tle > Select Paragraph Stylrs Editing 4. Prodiet the producti s) of the lollowing reactions, including stereochemistry when necessury und identify the mechanism of each substitution eaction (Syl vs SN2). product(s) type of reaction Br NaSMe DMSO Nal acetone HO Toxt Predicliong: On Accesiby. Irvestigate Enylish (United States) (99+ o search Pr ** FI1 FS & %23 6 7 2 3 R T. K L J F G C V Alt Alt LEarrow_forwardW AutoSave On chem - Compatibility Mode • Saved - O Search (Alt+Q) raghav grover RG File Home Insert Draw Design Layout References Mailings Review View Help P Comments A Share O Find Times New Roman v 12 - A A Aa v A Normal Body Text List Paragraph No Spacing E Replace Paste В I U ab x, x A - Dictate Editor Reuse A Select v Files Undo Clipboard s Font Paragraph Styles Editing Voice Editor Reuse Files Q6. Provide a step-wise synthesis for the following compound using benzene as a starting material. Br `NH2 K Accessibility: Unavailable D'Focus Page 4 of 13 523 words English (United States) 16 ENG 6:17 PM O Type here to search -16°C US 2022-03-02 近arrow_forwardin text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all working!!!!!!!arrow_forward
- W AutoSave On homewrok - Compatibility Mode - Saved - O Search (Alt+Q) raghav grover RG File Home Insert Design Layout References Mailings Review View Help O Comments A Share Draw O Find Times New Roman v 12 - A A Aa v A Normal Body Text List Paragraph No Spacing E Replace Paste В I U v ab x, x A - Dictate Editor Reuse A Select v Files Undo Clipboard s Font Paragraph Styles Editing Voice Editor Reuse Files 3 17. Provide a curved arrow mechanism for the following transformation. Br NaN3 N3 DMSO Page 17 of 19 1250 words English (United States) Accessibility: Unavailable D Focus 22 ENG 1:45 PM O Type here to search US 2021-12-16arrow_forwardPls help ASAParrow_forwardn Home Work-2 (page 7 of 8) b My Questions | bartleby A moodle.nct.edu.om/mod/quiz/attempt.php?attempt=709737&cmid=76257&page=6 E Apps * Bookmarks تحويل كيلومتر إلى م. .. © E Reading list NCT e-Learning Portal Courses Reports - e-Services Academic Departments - ETC - CIMS - Muayid Mahmood Mohammed Al Azri Fundamentals Of Chemistry (Engineering) Dashboard / My courses / CHEM1100 / Home works / Home Work-2 Quiz navigation Question 7 How many Faraday is needed to deposit 1.8 g of Sodium (Na) from NaCl solution using electrolysis process. Not yet 1 2 3 4 5 6 8 answered Answer: Marked out of Finish attempt . 1.00 P Flag question Time left 191:45:56 Previous page Next page - Submission Link for Home Work-1 Jump to. Quiz 1 for Section-3 - You are logged in as Muayid Mahmood Mohammed Al Azri (Log out) CHEM1100 Data retention summary. Get the mobile app 10:13 PM P Type here to search 72°F Clear O E 1 G 4) ENG 12/14/2021arrow_forward
- Select all true statements. ANFO explosive ...1-is more sensitive when density is higher than 1.2g/cm32-produces carbon monoxide (CO) when in a fuel shortage3-has a low velocity of detonation (VOD) and is useful to produce heave action forsoft and fractured rocks4-should not be stored for long periods of time5-has a higher velocity of detonation (VOD) when used in small diameter blast holes6-can be over-oiled (fuel excess) to account for potential fuel evaporation duringhot summer monthsarrow_forwardW AutoSave On chem - Compatibility Mode • Saving... O Search (Alt+Q) raghav grover RG File Home Insert Draw Design Layout References Mailings Review View Help P Comments A Share O Find Times New Roman v 12 - A A Aa - A Normal Body Text List Paragraph No Spacing E Replace Paste В I U v ab x, x A - Dictate Editor Reuse A Select v Files Undo Clipboard a Font Paragraph Styles Editing Voice Editor Reuse Files Q5. Provide a reasonable curved arrow mechanism for each of the following A. AICI3 Page 3 of 13 514 words English (United States) Accessibility: Unavailable D Focus 16 ENG 6:13 PM O Type here to search -16°C US 2022-03-02arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY