If the plant is to produce 176 tons of ethylene oxide per day, working in a steady state, calculate the flow rates of each component in the different streams of the system.
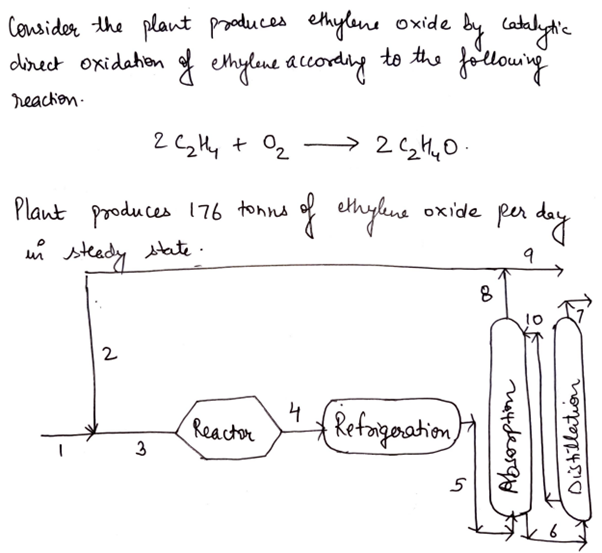
It is intended to build a plant (see figure) to produce ethylene oxide by catalytic direct oxidation of ethylene according to the reaction shown in the figure. The outlet stream from the reactor (4) is cooled and passes to an absorption column, where the ethylene oxide in water is completely absorbed and this mixture exits through the lower part of the column (6). The other compounds (without water or ethylene oxide) leave the upper part of the column (8). 90% (in moles) of this stream is recirculated (2) to the reactor and a purge (9) is established to avoid the accumulation of inerts. The mixture of water and ethylene oxide is taken (6) to a distillation train in which, on the one hand, the ethylene oxide (7) is separated and, on the other, the water, which is recirculated (10) to the absorption column. The feed (1) of the process is a mixture of industrial ethylene and air. Industrial ethylene contains 2% (by mole) of inert impurities. Air contains 21% oxygen and 79% nitrogen (moles). In working conditions, 5 tons of air have to be fed for each ton of ethylene oxide produced. Under these conditions, 55% of the ethylene moles reaching the reactor are transformed into ethylene oxide. If the plant is to produce 176 tons of ethylene oxide per day, working in a steady state, calculate the flow rates of each component in the different streams of the system.

Step by step
Solved in 7 steps with 7 images









