
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
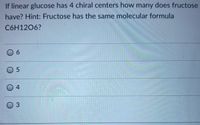
Transcribed Image Text:If linear glucose has 4 chiral centers how many does fructose
have? Hint: Fructose has the same molecular formula
C6H1206?
0 6
0 5
04
03
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- DHA is a fatty acid derived from fish oil and an abundant fatty acid invertebrate brains. Hydrogenation of DHA forms docosanoic acid[CH3(CH2)20CO2H], and ozonolysis forms CH3CH2CHO, CH2(CHO)2 (fiveequivalents), and HCOCH2CH2CO2H. What is the structure of DHA if all double bonds have the Z configuration?arrow_forwardIf the glucose molecule was labeled with 14C at the C-3, which carbon atom in pyruvate would you expect to find labeled? Select the structure of pyruvate showing its appropriate structure at pH 7.4. The structure should have a carbon highlighted in green and bold that corresponds to the original 14C labeled C-3 of glucose. H3C-C-C-OH H3C-C-C -O H3C-C-c-OH H3C C-C- H3C-C-C- H3C-C HO-arrow_forward1. (a) The diagram below shows a structure of monosaccharide. CH,OH C=0 H-C-OH НО—С—Н ČH,OH i. Draw the figure above on your answer sheet and indicate ALL chiral carbon(s) in your figure with *. ii. With reference to the number of carbon, the type of carbonyl group and D/L configuration, suggest a name consisting these three properties to describe the monosaccharide shown above. Explain why this monosaccharide is in D- or L- configuration as you suggested in (ii). 111. (b) The diagram below shows different configurations of glucose. CH,OH CHO ÇH,OH H H-C-OH O. OH H. H. OH H. H OH HO-C-H но H-C-OH но H. H-C-OH OH OH ČH,OH A В C i. Which glucose molecule, A or C, is in a configuration? Explain. ii. What is the name of the conversion among different configurations? iii. Glycosaminoglycans (GAG) consist of modified sugars. Give a name of the typical modification to the sugar. What makes GAG ideal for the fluid in the joints? toarrow_forward
- how do we name the bond between monomer B and monomer C?(anomeric designation‘s name plesse)arrow_forward"Potassium Bromate (KBr03) is added to flour as a softener that strengthens the texture of bread. It can do this by forming disulfide bonds between two cysteine amino acids in a reaction similar to the chemical equation given below. The function of KBr03 is to remove the 2 electrons needed to form the disulfide bond as shown in the figure below. Each of the cysteine amino acids will be in a protein chain, so the reaction binds the two chains together, giving the dough cohesion. However, potassium bromate is a proven carcinogen and is only allowed in foods in the United States because bakers will add the right amount and correct It has been assumed to meet the temperature and cooking time conditions. " KBRO, + C,H¸NO,SH → KBr + H,O+C,H„N,O,S, Balance the reaction of potassium bromate (KBr03) with the cysteine molecule (C3H6 NO2 SH) by converting it into the reduced step matrix using the additive matrix method. (Kis the symbol of potassium in the question) (Homogeneous linear equation…arrow_forwardConsider the following cyclic monosaccharide. CH₂OH H H H H OH HO OH Part 1 of 2 OH H Classify the monosaccharide as an aor ẞ isomer. Select the single best answer. ○ẞ isomer a isomer Part: 1/2 Part 2 of 2 Draw the other isomer of the cyclic form using a Haworth projection. Click and drag to start drawing a structure. ם' Garrow_forward
- Consider the structure shown below. он 3 5 CH; O CH, O H CH; O 1 H-N-CH,-C-N-CH;-C-N-CH-C-N-CH-C-N-CH-C-ơ 2 H. H H Fill in the blank with an integer (1, 2, 3, 4, 5..) as shown in the diagram or to represent a specified number. A hydrophilic side chain is indicated by the numberarrow_forwardcis, cis-9,12-OCTAdecadiENoic acid Enumerate the number of carbons and unsaturations (double bonds) in this moleculearrow_forwardHhhharrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON