
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
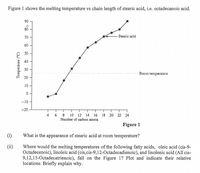
Transcribed Image Text:Figure 1 shows the melting temperature vs chain length of stearic acid, i.e. octadecanoic acid.
90
80
- Stearic acid
70
60
40
30
Room temperature
20
10
-10
-20
4 6 8 10 12 14 16 18 20 22 24
Number of carbon atoms
Figure 1
(i)
What is the appearance of stearic acid at room temperature?
(ii)
Where would the melting temperatures of the following fatty acids, oleic acid (cis-9-
Octadecenoic), linoleic acid (cis,cis-9,12-Octadecadienoic), and linolenic acid (All cis-
9,12,15-Octadecatrienoic), fall on the Figure 1? Plot and indicate their relative
locations. Briefly explain why.
Temperature ("C)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- The pka of the side chain of histidine is 6.00. What is the percent of protonated histidine at pH 6.4? 15.3% 28.5% 45.3% O 66.6%arrow_forwardDescribe the important biochemical properties of carbohydrates and why carbohydrates would be considered polar molecules. A▾ B I X @ F 7arrow_forwardLipoproteins transport dietary fats through the bloodstream and deliver them to the cells. Cholesterol (below) is mainly transported as a cholesterol ester via the condensation reaction of cholesterol and a free fatty acid. Draw the structure of the cholesterol ester produced from the condensation of cholesterol and stearic acid (structures here Ł). НО םבי יarrow_forward
- "Potassium Bromate (KBr03) is added to flour as a softener that strengthens the texture of bread. It can do this by forming disulfide bonds between two cysteine amino acids in a reaction similar to the chemical equation given below. The function of KBr03 is to remove the 2 electrons needed to form the disulfide bond as shown in the figure below. Each of the cysteine amino acids will be in a protein chain, so the reaction binds the two chains together, giving the dough cohesion. However, potassium bromate is a proven carcinogen and is only allowed in foods in the United States because bakers will add the right amount and correct It has been assumed to meet the temperature and cooking time conditions. " KBRO, + C,H¸NO,SH → KBr + H,O+C,H„N,O,S, Balance the reaction of potassium bromate (KBr03) with the cysteine molecule (C3H6 NO2 SH) by converting it into the reduced step matrix using the additive matrix method. (Kis the symbol of potassium in the question) (Homogeneous linear equation…arrow_forwardIdentify the components of an amino acid in the following diagram. (a) ______________ (b) ______________ (c) ______________ (d) ______________arrow_forwardThe figure below illustrates the molecular structures of two fatty acids. A B H₂C The structural formula of erucic acid and behenic acid с H₂C D erucic acid behenic acid Which of the following best explains why erucic acid is liquid at room temperature but behenic acid is solid at room temperature? O OH The presence of a double carbon to carbon bond in erucic acid prevents the molecule from packing closely together. The lack of any double carbon-carbon bonds in behenic acid causes the molecule to be come polar and therefore packed more tightly. The larger number of carbon atoms in erucic acid prevents the molecule from packing tightly together. OH The smaller number of carbon atoms in behenic acid creates stronger covalent bonds between the carbon atoms allowing for them to pack more tightly together.arrow_forward
- Draw the structures of the amino acids formed at physiological pH when the tripeptide below is hydrolyzed. H H O H H O H H O | + || H -N N H HC. CH2 CH2 HC H₂C H₂C OH HC CH CH NH₂ Click and drag to start drawing a structure.arrow_forwardb) The likelihood of both triglycerides and phospholipids to behave as liquids at a given temperature is affected by their degree of saturation. Explain what saturation is and provide a biochemical explanation for why it affects the likelihood of a lipid to behave as a liquid at a given temperature. 5) a) Proteins have multiple "levels" of structural complexity. Match the type of chemical bond on the left with the level of protein structure that they are specifically involved in maintaining on the right. (Note that more than one letter may apply to each structural level and that each letter may be used more than once or not at all). a. disulphide bonds Tertiary structure b. hydrogen bonds Primary structure c. ionic bonds Quaternary structure d. peptide bonds Secondary structurearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON