
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
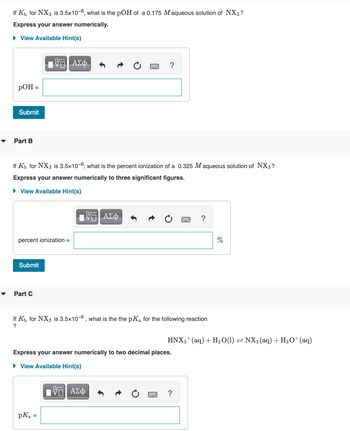
Transcribed Image Text:If K₁ for NX3 is 3.5x10-6, what is the pOH of a 0.175 M aqueous solution of NX3?
Express your answer numerically.
► View Available Hint(s)
pOH =
Submit
Part B
If Kb for NX3 is 3.5x10-6, what is the percent ionization of a 0.325 Maqueous solution of NX3?
Express your answer numerically to three significant figures.
► View Available Hint(s)
percent ionization =
Submit
ΤΙ ΑΣΦ
Part C
15| ΑΣΦ
pKa =
?
If K₁ for NX3 is 3.5x10-6, what is the the pKa for the following reaction
?
Express your answer numerically to two decimal places.
► View Available Hint(s)
Π ΑΣΦ
?
HNX3+ (aq) + H₂O(1) ⇒ NX3 (aq) + H3O+ (aq)
=
?
%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following solutions has a pOH > 7? Group of answer choices 0.01 M NH4NO3 0.01 M NaNO3 0.01 M KNO2 0.01 M KNO3arrow_forwardWhat is [H+] in a 0.470 M solution of acrylic acid, CH2CHCOOH (Ka = 3.16 x 10-5)? 13arrow_forward10) Find the p H of a 0.291 M N a A solution. The salt completely dissociates into N aplusand Aminus. N a is the cation of a strong base so it will not affect the p H of the solution. Aminus is the anion of the weak acid H A. The K a of H A is 1.9E-5. If the 5% rule is valid you may apply it.arrow_forward
- Calculate the concentration of all species in a 0.22 molL-1 KNO2 solution. Ka(HNO2)=5.6x10-4 Express your answers in moles per litre to two significant figures separated by commas. [K+], [NO2-], [HNO2], [OH-], [H3O+] =arrow_forwardDetermine the percent ionization of a 0.215 M solution of benzoic acid. Express your answer using two significant figures. Hν ΑΣφ ? %arrow_forwardThe hydroxide ion concentration, [OH-], of an aqueous solution of 0.463 M isoquinoline (a weak base with the 2.5x10-⁹, is: formula C₂H₂N), K₁ [OH-] = M. =arrow_forward
- After dissolving NaOH in water, the final concentration of OH is 1.27x10-4. What is the pOH of this solution? Give your answer to the 2nd decimal.arrow_forwardCalculate [H3O*] in the following aqueous solution at 25°C: [OH¯]=1.9x10-9 M . Express your answer using two significant figures. V ΑΣφ [H3O+] = Submit Request Answer Part B Calculate [H30+] in the following aqueous solution at 25 ° C: [OH-] = 2.9x10-2 M. Express your answer using two significant figures. ΑΣφ [H3O*] = Submit Request Answerarrow_forwardCalculate H30" of the following polyprotic acid solution: 0.130 M H2CO3 (Kal 4.3 × 10-7, K2 = 5.6 x 10 11). Express your answer using two significant figures. [H3O*] = Marrow_forward
- A 0.149 mol L-¹ solution of a monoprotic acid has a percent ionization of 2.74%. ▾ Part A Determine the acid ionization constant (Ka) for the acid. Express your answer to three significant figures. VE ΑΣΦ K₁ = Ka Submit Previous Answers Request Answer X Incorrect; Try Again I ?arrow_forwardPlease re-create the table and rank each solution 1 (lowest) to 4 (highest). Thank you!arrow_forwardOrder these chemical species by increasing pH of an 0.1 M aqueous solution of each. That is, imagine making an 0.1 M solution of each species. Select 1 next to the species that makes the solution with the lowest pH. Select 2 next to the species that makes the solution with the next higher pH, and so on. Notice that some of the rankings have been filled in for you already. Also notice that water is on the list. For that particular case, just compare the pH of pure water to the pH of the other solutions. Note for advanced students: for all charged species, you may assume the necessary counterions act as neither acids nor bases. relative pH of 0.1 M aqueous solution species НСООН 4 HC,04 (Choose one) ▼ H,O 1 (lowest) HNO, (Choose one) ▼arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY