
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
![Part A
-1
Calculate the [H₂O¹] of a 0.897 mol L citric acid (H₂CeHsO7) solution. (Kat = 7.4 x 10-¹, K₁2 = 1.7 x 10-5)
Express your answer to two significant figures.
| ΑΣΦ
[H₂O¹]=
Submit Previous Answers Request Answer
X Incorrect; Try Again
Part B
Calculate the pH of this solution.
Express your answer to two decimal places.
pH =
ΑΣΦ
?
mol L-1](https://content.bartleby.com/qna-images/question/4f1ad8cd-79a4-4259-96a6-1ecf688cef1d/b55fe6d0-87eb-4784-8ad7-e623e3abc60a/d9vdf2_thumbnail.jpeg)
Transcribed Image Text:Part A
-1
Calculate the [H₂O¹] of a 0.897 mol L citric acid (H₂CeHsO7) solution. (Kat = 7.4 x 10-¹, K₁2 = 1.7 x 10-5)
Express your answer to two significant figures.
| ΑΣΦ
[H₂O¹]=
Submit Previous Answers Request Answer
X Incorrect; Try Again
Part B
Calculate the pH of this solution.
Express your answer to two decimal places.
pH =
ΑΣΦ
?
mol L-1
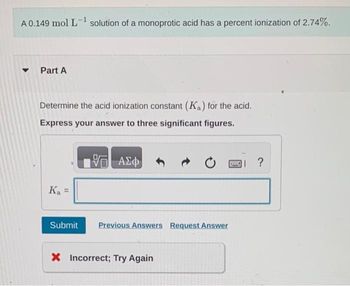
Transcribed Image Text:A 0.149 mol L-¹ solution of a monoprotic acid has a percent ionization of 2.74%.
▾ Part A
Determine the acid ionization constant (Ka) for the acid.
Express your answer to three significant figures.
VE ΑΣΦ
K₁ =
Ka
Submit
Previous Answers Request Answer
X Incorrect; Try Again
I ?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 13 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For a certain acid pK = 6.59. Calculate the pH at which an aqueous solution of this acid would be 0.36% dissociated. Round your a answer to 2 decimal places. pH = X 3arrow_forwardComplete the table below. Round each of your entries to 2 significant digits. You may assume the temperature is 25 °C. conjugate acid formula conjugate base Ка formula Ко ✗ HSO 0.012 ☐ ☐ -4 CH3NH2 4.4 × 10 ☐ CHN 1.7 x 10 -9 ☐ x10arrow_forwardDetermine the [H3O+][H3O+] of a 0.160 MM solution of benzoic acid. Express your answer using two significant figures. Determine pHpH of this solution of benzoic acid. Express your answer to two decimal places.arrow_forward
- ACIDS AND BASES Calculating the pH of a weak base solution -8- The base protonation constant K, of lidocaine (CH, NONH) is 1.15 x 10 °. 14 Calculate the pH of a 0.30 M solution of lidocaine at 25 °C. Round your answer to 1 decimal place. pH =arrow_forwardBha Please don't provide the handwriting solutionarrow_forwardStuck on this :(arrow_forward
- Calculate the pH of a 0.971 M solution of ammonia, NH3, given that Kb = 1.80 × 10–5. Provide your answer to three decimal places. Do not enter units.arrow_forwardComplete the table below. Round each of your entries to 2 significant digits. You may assume the temperature is 25 °C. conjugate acid conjugate base formula HNO₂ K Ко formula ☐ 4.5 × 10 4 K₁₂ × -5 NH3 1.8 × 10 -7 HS 1.8 × 10 G x10arrow_forwardCalculate the pH of a 0.774 M solution of ammonia, NH3, given that Kb = 1.80 × 10–5. Provide your answer to three decimal places. Do not enter units.arrow_forward
- What is the pH of a 0.370 M aqueous solution of methylammonium chloride, CH3NH3Cl? The Kb of methyl amine, CH3NH2 is 4.4x10-4. You do not need to solve the quadratic equation for this problem. Express your answer to two decimal places.arrow_forward-8 Calculate the pH of a 4.9 x 10 M aqueous solution of hydroiodic acid (HI). Round your answer to 2 decimal places.arrow_forwardIf a sample of a certain solution is determined to have a pH of 3.7, what is the concentration of [H3O+] in the solution? Submit your answer in scientific notation and round off the significand (first number) of your answer to two decimal places. Answer x10 moles/liter Keypad Keyboard Shortcutsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY