
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
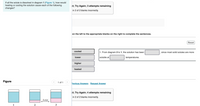
Transcribed Image Text:If all the solute is dissolved in diagram 1 (Figure 1), how would
heating or cooling the solution cause each of the following
changes?
ct; Try Again; 3 attempts remaining
in 2 of 2 blanks incorrectly.
on the left to the appropriate blanks on the right to complete the sentences.
Reset
cooled
1. From diagram 2 to 1, the solution has been
since most solid solutes are more
lower
soluble at
temperatures.
higher
heated
Figure
1 of 1
>
Previous Answers Request Answer
ct; Try Again; 4 attempts remaining
in 2 of 2 blanks incorrectly.
Solid
3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Part C. Solubility versus Temperature 1. (a) Which solution(s) containing 1.0 g of salt (NaCl and NH,CI are both considered "salts") per 5 ml. of water is (are) unsaturated and why? 2. (a) Temperature of each solution before heating: NaCl: NH,Cl: (b) Which solution(s) containing 2.4 g total of salt per 5 ml of water is (are) saturated at room temperature and why? 3. (a) Temperature of each solution in the hot water bath: NaCl: NH.CI: (b) Which salt is least soluble at elevated temperatures? (c) Classify the salt solutions at elevated temperatures as either saturated or unsaturated. NaCl: NH.CI: 4. (a) Did the salt solution(s) that were unsaturated at the elevated temperatures become saturated when cooled? How do you know? 6.arrow_forwardWhen 0.258 g of a unknown molecular compound, was dissolved in 40.0 g of benzene, the freezing point of the solution was lowered to 5.23 °C. What is the molecular weight of the unknown compound? Kf benzene = 5.12 °C/m. Tf = 5.5 °C. * solve with steps and explains and equations please??arrow_forwardThe second picture are the answers choicesarrow_forward
- ne > My courses > 1802 > Topic 1 Final Exam What is the molality of NaCl in a solution that is 2.5 M NaCl, with a density of 1.02 g/mL? formual mass of NaCl = 58.5 g/mol of PREVIOUS ACTIVITY second exam Type here to searcharrow_forwardThe solubilities of three salts in water are shown in the graph. For each salt, determine how much will remain undissolved if 500 g is mixed into a liter of pure water at 40 °C. One significant figure is sufficient in each answer. Pb(NO3)2: KC1: K₂Cr₂O7: OD g 6.D Solubility (g/L) 1000 900 800 700 600 500 400 300 200 100 Pb(NO3)2 K₂Cr₂O, КСІ 0 10 20 30 40 50 60 70 80 90 100 Temperature (℃)arrow_forwardCan you please type out the answer, don’t write it on paper!arrow_forward
- 3. Why is molality and not molarity used to express concentration in the equations for freezing point depression and boiling point elevation?arrow_forwardplease help with the following questiongarrow_forwardArrange the boiling points of the aqueous solutions, relative to pure water. Assume complete dissociation for the ionic compounds. Highest boiling point Lowest boiling point Answer Bank 0.17 m C„H„011 0.13 m MgCl, 0.16 m NaNO3 0.24 m C,H„011 H,O 22 (12arrow_forward
- In which solvent would the following solute be most likely to dissolve? H. O H-ö-H .. H O H H-C-C-Ç-H H H H HH H H H-C-C-Ç-C-C-Ç-H H H H H H H H. H-C-OH I-O-I I-O-I I I-Ú- I I-Ú -Iarrow_forwardWhy does freezing point decrease and boiling point increase when solute is added? Please answer in not less than 3 sentences.arrow_forwardplease do 9 and 10arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY