
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:ne >
My courses > 1802 > Topic 1 Final Exam
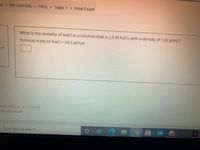
What is the molality of NaCl in a solution that is 2.5 M NaCl, with a density of 1.02 g/mL?
formual mass of NaCl = 58.5 g/mol
of
PREVIOUS ACTIVITY
second exam
Type here to search
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- An aqueous solution of 1.00 M ammonia (NH3) has a density of 0.900 g/mL. What is its concentration in molality (mol/kg)?arrow_forwardWhat is the molality of a solution produced by dissolving 11.70g of LiCl (42.39 g/mol) in water to make 0.115L of solution with a density of 1.102 g/mL?arrow_forward9. What is the molality of a solution of 34.0 g of acetone (CH3COCH3) in 300.0 mL water (mw of ethanol =58.1 g/mol)?A 1.37 m B 1.65 m C 2.27 m D 2.61 m E 1.95 marrow_forward
- Your pure water boils at 100.00 C. You add some sugar into it and now it boils at 101.20C. What is the molality? Kb= 0.512 Ckg/molarrow_forwardWhat is the molal concentration of C10H8 in a solution made by dissolving 0.127 g C10H8 in 9.132 g cyclohexane?arrow_forwardWhat is the molality of a 32.5% HCIO4 solution? The density of this solution is 1.67 g/mL. 5.40 m 16.6 m 7.25 m 48.0 m 4.79 marrow_forward
- Calculate the molality of a solution formed by adding 2.60 g NHCl to 19.1 g of water. molality: m NH₂Clarrow_forwardCalculate the molality of a solution formed by adding 2.50 g NH4Cl to 16.7 g of water.arrow_forwardWhat is the molality of a solution made by dissolving 25.9 grams of CaCl 2 in 625 grams of water? 0.747 m 10.5 m 0.00594 m 0.00686 m 0.373 marrow_forward
- An aqueous solution of hydrofluoric acid is 30.0% HF, by mass, and has a density of 1.101 g cm³. What are the molality and molarity of HF in this solution? 70.02mol and 40.2kg O 1.499mol and 21.4kg O 5.80mol and 32.0kg Oarrow_forwardWhat mass of H2O is needed to dissolve 4.69 g KMnO4 to produce a solution having a molality of 0.299 m? Assume that the density of the solution is 1.00 g/mL. The molar mass of KMnO4 is 158.03 g/mol and the molar mass of H2O is 18.02 g/mol.arrow_forwardWhat is the molality (mol/kg) of a solution prepared by dissolving 34 g of Calcium Chloride (CaCl2) in 250 mL of water? Assume water has a density of 1.0000 g/mL and the volume does not change. Hint: CaCl2 molar mass = 110.98 g/mol 4.8 m 3.6 m 2.4 m 1.2 marrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY