Question
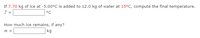
Transcribed Image Text:If 7.70 kg of ice at -5.00°C is added to 12.0 kg of water at 15°C, compute the final temperature.
T =
°C
How much ice remains, if any?
m =
kg
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- One end of a copper rod is kept at 10°C while the other end is kept at 10'C, Find the rate of conduction through the rod if the diameter of the rod is 4.8cm and the length of the rod is 1.5m. O 20 cal/s O 100 cal/s O 10 calis O 2 cal/sarrow_forwardWhat is the change in length of a 3.00-cm-long column of mercury if its temperature changes from 37.0ºC to 40.0ºC , assuming the mercury is unconstrained?arrow_forward8. (a) If a rod of brass 0.35 m 15 to 85°C while its ends are maintained rigid long is heated from determine the type and magnitude of stress that develops Assume that at that at 95°C the rod is stress free. 1 (b) Also calculate the stress magnitude if a rod Im long is used . [ Linear coeff. of brass = 20.0 x10 6/2]arrow_forward
- A wire is 24.5 m long at 2.50°C and is 1.37 cm longer at 35.5°C. Find the wire's coefficient of linear expansion (in (°C)-1). HINT |(°C)-1 Need Help? Read It Watch Itarrow_forwardA 15.9 L aluminum pot filled to the brim with 7.85˚C water. How many litres of water will overflow when the temperature of the water is raised to 76.96˚C [round your final answer to three decimal places]? Note that the pot and the water will be in equilibrium at both temperatures. {volume coefficient of thermal expansion for water = 207 × 10-6 ˚C-1; volume coefficient of thermal expansion for aluminum = 70 × 10-6 ˚C-1}arrow_forwardIce of mass 50.0 g at -10.0° C is added tp 200 g of water at 15.0° C in a 100 g glass container of specific heat 0.200 cal/g-C° at an initial temperature of 25.0° C. Find the final temperature of the system.arrow_forward
- What mass of steam at 100◦C must be mixed with 150 g of ice at −60.0◦C, in a thermally insulated container, to produce liquid water at 50◦C?arrow_forwardA glass louvre pane 0.4 cm thick has an area of 2×104cm2 . On a very cold day the temperature difference between the inside and outside surfaces of the pane is 25 °C. determine the rate of heat flow through this window. [ K glass = 0.837 Wm-1 °C ]arrow_forwardThe boiling point of ethyl alcohol is 351 K at atmospheric pressure. (a) What is this temperature on the Celsius scale? |°C (b) What is this temperature on the Fahrenheit scale? | °Farrow_forward
arrow_back_ios
arrow_forward_ios